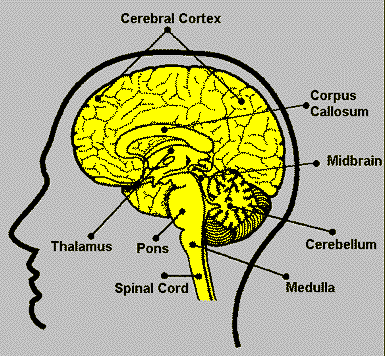
| Brain Structures |
Function |
From the excellent
website: Neuroscience
for Kids |
Cerebral
Cortex |
* Thought
* Voluntary movement
* Language
* Reasoning
* Perception |
The word "cortex" comes from the
Latin word for "bark" (of a tree). This is because
the cortex is a sheet of tissue that makes up the outer layer
of the brain. The thickness of the cerebral cortex varies from
2 to 6 mm. The right and left sides of the cerebral cortex are
connected by a thick band of nerve fibers called the "corpus
callosum." In higher mammals such as humans, the cerebral
cortex looks like it has many bumps and grooves. A bump or bulge
on the cortex is called a gyrus (the plural of the word gyrus
is "gyri") and a groove is called a sulcus (the plural
of the word sulcus is "sulci"). Lower mammals, such
as rats and mice, have very few gyri and sulci. |
| Cerebellum |
* Movement
* Balance
* Posture |
The word "cerebellum" comes from
the Latin word for "little brain." The cerebellum
is located behind the brain stem. In some ways, the cerebellum
is similar to the cerebral cortex: the cerebellum is divided
into hemispheres and has a cortex that surrounds these hemispheres.
|
| Brain stem |
* Breathing
* Heart Rate
* Blood Pressure |
The brain stem is a general term for the area
of the brain between the thalamus and spinal cord. Structures
within the brain stem include the medulla, pons, tectum, reticular
formation and tegmentum. Some of these areas are responsible
for the most basic functions of life such as breathing, heart
rate and blood pressure. |
| Hypothalamus |
* Body Temperature
* Emotions
* Hunger
* Thirst
* Circadian Rhythms |
The hypothalamus is composed of several different
areas and is located at the base of the brain. Although it is
the size of only a pea (about 1/300 of the total brain weight),
the hypothalamus is responsible for some very important functions.
One important function of the hypothalamus is the control of
body temperature. The hypothalamus acts as a "thermostat"
by sensing changes in body temperature and then sending signals
to adjust the temperature. For example, if you are too hot,
the hypothalamus detects this and then sends a signal to expand
the capillaries in your skin. This causes blood to be cooled
faster. The hypothalamus also controls the pituitary. |
| Thalamus |
* Sensory processing
* Movement |
The thalamus receives sensory information and
relays this information to the cerebral cortex. The cerebral
cortex also sends information to the thalamus which then transmits
this information to other areas of the brain and spinal cord. |
| Limbic System |
* Emotions |
The limbic system (or the limbic areas) is
a group of structures that includes the amygdala, the hippocampus,
mammillary bodies and cingulate gyrus. These areas are important
for controlling the emotional response to a given situation.
The hippocampus is also important for memory. |
| Hippocampus |
* Learning
* Memory |
The hippocampus is one part of the limbic system
that is important for memory and learning. |
| Basal Ganglia |
* Movement |
The basal ganglia are a group of structures,
including the globus pallidus, caudate nucleus, subthalamic
nucleus, putamen and substantia nigra, that are important in
coordinating movement. |
| Midbrain |
* Vision
* Audition
* Eye Movement
* Body Movement |
The midbrain includes structures such as the
superior and inferior colliculi and red nucleus. There are several
other areas also in the midbrain. |
A
little background on GABA
Synthesis, storage and release
GABA was identified in the mammalian brain in 1950's. It
is believed to be the major inhibitory neurotransmitter
in the brain. It is this role which is of interest to the
neuropsychiatrist. GABA is synthesized from Glutamate. The
marker enzymeis Glutamic acid decarboxylase (GAD). GAD is
a pyridoxal cofactor dependent enzyme. A congential form
of B-6 vitamin deficiency is known to predispose to seizures
which are B-6 responsive. Glutamate is a pivotal amino acid
in the brain. It is dervied from alpha keto glutarate which
is one of the intermediates in the Krebs cycle by way of
the addition of an amine group. Glutamate also undergoes
transamination to form glutamine by addition of another
amine group. Glutamine then proceeds to the liver where
it is deaminated to regenerated glutamate which then returns
to the brain. This is brain's nitorgen cycle. In situations
where the liver is unable to deaminate the glutamine the
brain must obtain glutamate by draining the Kreb's cycle
intermediates. This in turn begins to impair cerebral energy
metabolism.
Following release GABA can be taken back up by the neurons
or by astrocytes. It appears that the release of GABA is
also under autoreceptor control. GABA is metabolized by
the enzym GABA transaminase (GABA-T) to form succinic acid
semialdehyde. Succinic acid semialdehyde is metabolized
further to form succinic acid which is also a Kreb's cycle
intermediate. GABA-T is inhibited by valproic acid. This
is the basis for the belief that valproic acid is GABAergic.
There are other alternative pathways for GABA metabolism.
There
are two basic subtypes, GABA-a and GABA-b.
GABA-a is
the most prevalent in the mammalian brain. The GABA-a receptor
is similar to acetylcholine receptor in that it is related
to an ion channel. In the case of GABA-a it is the chloride
ionophore. Binding of GABA to this receptor increases the
permeability to chloride ion which causes a hyperpolarization
of the neuron or inhibition. The GABA-a receptor has four
basic subunits, 2-alpha and 2 beta peptides which surround
a chloride channel. There are three basic binding sites
on this complex. The first is the GABA site. The second
is a benzodiazepine site. The third is in the channel and
is essentially a barbiturate site ...
The
GABA-b receptor is
a G-protein related receptor which is distinct from the
GABA-a sites. The highest concentrations of GABA-b receptors
is in the interpeduncular nuclie and cerebellum. It appears
that one of its prinicple effects is to increase the efflux
of K+ from the cell. This would result in a hyperpolarization.
Pharmacologically baclofen is considered a GABA-b agonist.
The principle effect of GABA-b agonism is muscle relaxation.
A significant relationship of dopamine and GABA exists.
In general GABA acts to reduce the firing of the dopaminergic
neurons in the tegmentum and substantia nigra. It forms
the basis for the use of benzodiazepines as augmentation
strategies in the treatment of psychosis. In addition benzodiazepines
may be helpful in cases where there is an over activity
of dopamine in the motor striatum such as Huntington's Chorea
or Tardive Dyskinesia. It is believed that they act by increasing
the feedback inhibition. The feedback inhibition from the
GABA neurons of the globus pallidus and putamen to the dopaminergic
neurons of the substantia nigra is an important modulating
force on the activity of the dopamine neurons.
Ref:
http://www.unifr.ch/biochem/DREYER/Neurotransmitters/gaba.htm
|
The
use of high doses increases the likelihood that potentially
significant toxic effects will be identified. Findings of
adverse effects in any one species do not necessarily indicate
such effects might be generated in humans. From a conservative
risk assessment perspective however, adverse findings in
animal species are assumed to represent potential effects
in humans, unless convincing evidence of species specificity
is available.
--
Food and Agricultural Organization of the United Nations
|
Note:
This is not an exhaustive list.
When time allows more information will be added.
Fentrifanil
-
Acaricide - CAS No. 62441-54-7
PubMed abstract: Observations
on 2'-chloro-2,4-dinitro-5',6-di(trifluoromethyl)-diphenylamine-induced
edema in the white matter of the
central nervous system of the rat.
Ref: Lock EA, Scales MD, Little RA. Toxicol
Appl Pharmacol 1981 Aug;60(1):121-30.
http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7281170&dopt=Abstract
Fipronil
- Acaricide,
Insecticide
- CAS No. 120068-37-3
The NOEL for developmental
toxicity is 0.5 ppm (0.05 mg/kg/day). The developmental neurotoxicity
LOEL is 200 ppm (15 mg/kg/day) based on: Decreased auditory startle
response; reduced swimming direction scores, group mean angle
measurements, and water ``Y'' maze times trails; and decreased
absolute-brain weights. The NOEL for developmental neurotoxicity
is 10 ppm (0.90 mg/kg/day).
Ref: Federal Register. July 17, 1998. Fipronil;
Pesticide Tolerance. Final Rule.
http://www.fluoridealert.org/pesticides/fipronil.fr.july.17.1998.htm
Fipronil
is the first phenylpyrazole insecticide introduced for pest control.
Although fipronil is known to inhibit GABA receptors, the detailed
mechanism of action remains to be seen. In order to elucidate
the mechanism of fipronil interaction with the mammalian GABAA
system, single-channel patch clamp experiments were performed
using rat dorsal root ganglion neurons. The amplitude of main
conductance state (27pS) current was not significantly altered
by co-application of 10 microM fipronil and 10 microM GABA. The
histograms of open time distribution were fitted to a sum of three
exponential functions. After application of 10 microM fipronil,
the proportion of the fastest component increased slightly and
that of the slowest component decreased slightly. Thus, the mean
open time was decreased from 11.4 ms to 7.8 ms by fipronil. The
histograms of closed time distribution were fitted to a sum of
four exponential functions. Fipronil 10 microM prolonged the slowest
time constant resulting in a prolongation of the mean closed time
from 29.7 ms to 52.8 ms. Thus, the frequency of channel openings
was reduced. Thus, the fipronil suppression
of GABA-induced whole-cell currents is caused in part by decreases
in the channel open time and the frequency of channel openings.
Ref: Pest
Manag Sci. 2004 May;60(5):487-92. Fipronil modulation of GABAA
receptor single-channel currents; by Ikeda T, Nagata K, Kono Y,
Yeh JZ, Narahashi T.
http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11052715&dopt=Abstract
Excerpt from absract:
The long-term objective is to define the fundamental basis for
the selective toxicity of insecticides acting
at the gamma-aminobutyric acid (GABA) receptor of mammals and
insects. This is the target of major neurotoxic insecticides acting
as both blockers and activators of the GABA-gated chloride channel.
More than 5,000,000,000 pounds of these channel blockers have
been used for pest control in the past 50 years and they range
in chlorine content from 52-73%. The major channel blockers used
at present, representing 6% of the insecticide market, are endosulfan
and lindane and this market share will increase
with expanded use of the newly-commercialized polyhalogenated
fipronil....
Ref: GABAERGIC
INSECTICIDE TOXICOLOGY; by CASIDA JE. From
Toxline at Toxnet. 2002. Supporting Agency: U.S. DEPT. OF HEALTH
AND HUMAN SERVICES; PUBLIC HEALTH SERVICE; NATIONAL INSTITUTES
OF HEALTH, NATIONAL INSTITUTE OF ENVIRONMENTAL HEALTH SCIENCES.
(See full abstract at
Toxline or
http://www.fluorideaction.org/pesticides/fipronil.abstracts.htm
The
long-term goal of the proposed study is to
elucidate the mechanism by which neuroactive insecticides exert
their toxic actions ... In
order to elucidate the physiological mechanisms of selective toxicity,
patch clamp data on the kinetics of receptors/channels and those
of insecticide modification will be compared between rat and cockroach
neurons for fipronil modulation of GABA
receptors, imidacloprid modulation of neuronal nicotinic
acetylcholine receptors (nnAChRs) ...
Ref:
Mode of
Action of Insecticides: Electrophysiological; by NARAHASHI
T. 2002. Supporting Agency: U.S. DEPT. OF HEALTH AND HUMAN SERVICES;
PUBLIC HEALTH SERVICE; NATIONAL INSTITUTES OF HEALTH, NATIONAL
INSTITUTE OF NEUROLOGICAL DISORDERS AND STROKE.
(See
full abstract at
Toxline or
http://www.fluorideaction.org/pesticides/fipronil.abstracts.htm
Flonicamid
- Insecticide - CAS No. 158062-67-0
“IKI-220
Technical: Combined Chronic Toxicity and Carcinogenciity Study
in Rats,” (M. Kuwahara; IET 98-0142; 8/16/04). ... This
volume contains discussion of questions concerning the bone/bone
marrow effects and cerebellar granular tumors
observed in the original study.... In this document the U.S. EPA
also questioned whether the cerebellar granular
cell tumors (observed histopathologically) were treatment related.
It was reported that GCT involving the meninges of the brain is
the most frequently reported benign neoplasm occurring in many
strains of rat. Historical controls obtained from 38 2-year studies
in Wistar rats (1981 - 2000) at RCC Ltd., Switzerland showed a
range of 0 - 7.14% (M) and 0 - 4.35% (F). The report concluded
that since GCTs of meninges are observed only microscopically,
small ones may be included in the sections examined only by chance.
The tumors do not metastasize and are possibly underreported.
Thus their true incidence is not really known. The report considered
these observations to be incidental and unrelated to treatment.
DPR considers the GCTs to be marginally
increased in females when compared to historical controls but
essentially equivocal. (Silva, 4/11/05)
Reference:
April 28, 2005 - Summary of toxicology data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/flonicamid.ca.epa.2005.pdf
Fluazifop-butyl
- Herbicide - CAS No. 69806-50-4
Fluazifop butyl was
evaluated for reproductive and developmental effects in 2 successive
generations of Charles River Wistar strain rats (30/sex/group)
exposed continuously to 0, 10, 80 and 250 ppm in the diet. Each
respective parent generation had received treatment for a minimum
of 100 days (F0) and 120 days (F1) prior to mating. F0 and F1
dams weaned their progeny for 25 days postpartum to time of offspring
selection for mating and continued study (F1) or sacrifice (F1,
F2). Thirty days after sacrifice of their offspring, the surviving
F1 females and select F1 males were sacrificed and with representative
F1 and F2 offspring were examined histologically... .
Testis and epididymis weights in the males (80, 250 ppm), and
pituitary gland (80, 250 ppm), uterus (80, 250 ppm),
brain (250 ppm) and lung weights (250 ppm)
in females were significantly reduced...
[ICI AMERS INC; Fluazifop butyl:
Effects Upon Reproductive Performance of Rats Treated Continuously
Through 2 Generations (Final Report); 03/17/81; EPA Doc No. 88-920006849;
Fiche No. OTS05543854]
Ref: TOXNET profile from Hazardous Substances
Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/fluazifop-butyl.toxnet.hsdb.htm
Fluazinam
-
Fungicide
- CAS
No. 79622-59-6
-- In subchronic and
chronic toxicity studies, fluazinam targeted the following organs:
liver, lung, uterus, testes, pancreas, thymus, thyroid, stomach,
eyes and brain...
-- In acute and subchronic neurotoxicity studies, treatment with
fluazinam resulted in marked neuropathology
in the form of vacuolation of the white matter of the brain.
Special
studies have been submitted that show this effect is not due to
fluazinam itself, but rather to a manufacturing impurity.
In the subchronic study, generalized toxicity was observed as
decreased body weight and food efficiency. A developmental neurotoxicity
study was suggested upon preliminary review of the data and the
applicant submitted a rationale. Upon full review of the dataset,
a developmental neurotoxicity study is a
requirement for full registration. Special
studies were generated to determine the cause of the white matter
vacuolation. All
nine impurities were tested at doses that reflected their relative
content in the technical product. Only one
impurity was found to produce the vacuolation effects and all
subsequent special studies focus on this chemical. The vacuolation
was determined to be in the myelin sheaths surrounding axons in
the white matter. Dogs, mice and rats were found to have comparable
results when tested in similar manners. Older animals were found
to be more susceptible than the young. All vacuolation
effects were found to be reversible. Neurotoxic findings were
common at high doses and included decreased motor activity, partial
paralysis and ataxia. This effect was observed at high doses (LOAEL
was 50 mg/kg/d) when the dose level of impurity no. 5 was sufficiently
high to cause these effects. Therefore, the
presence of this impurity will be limited to 0.1% of the technical
active.
-- Determination of acceptable daily intake
(ADI). The recommended ADI for fluazinam is 0.0011 mg/kg bw/day.
The chronic toxicity and oncogenicity study in mice was considered
the most appropriate study to assess chronic dietary exposure.
The no observed adverse effect level (NOAEL) was 1.1 mg/kg bw/day,
based on increased incidences of brown pigmented macrophages in
the liver, increased incidences of eosinophilic vacuolated hepatocytes
in males and increased liver weights relative to body weights.
The standard uncertainty factor of 100-fold is applied to account
for intraspecies and interspecies variability, an additional 10-fold
safety factor is recommended to firstly, protect for endocrine-related
effects and secondly, to account for the lack of a developmental
neurotoxicity (DNT) study. This provides
margins of safety of 9100 to the NOEL for white matter vacuolation
and 6350 to the NOEL for developmental effects in the rabbit developmental
study.
-- The USEPA has chosen an ADI of 0.00367 mg/kg bw/d based on
a 2-year carcinogenicity study in mice (1.1 mg/kg bw/d with 100-fold
uncertainty factor (UF) and three-fold Food Quality Protection
Act (FQPA) safety factor.) They have also
requested a developmental neurotoxicity study.
Ref:
Canada: Regulatory
Note REG2003-12. Fluazinam. Pest
Management Regulatory Agency. Health Canada. Ottawa. October 27,
2003.
http://www.fluorideaction.org/pesticides/fluazinam.canada.report2003.pdf
90-Day oral toxicity
dogs NOAEL = 10 mg/kg/day LOAEL = 100 mg/kg/day based on retinal
effects, increased relative liver weight, liver histopathology
and possible increased serum alkaline phosphatase in females and
possible marginal vacuolation of the cerebral
white matter (equivocal)...
Carcinogenicity mice NOAEL = Males:<126 mg/kg/day, Females: <162
mg/kg/day LOAEL = Males: 126 mg/kg/day; Females: 162 mg/kg/day
based on increased liver weights and liver and brain
histopathology in both sexes
Special study: 4-Week dietary (Range-finding) mice NOAEL = not
identified (Males; <555 mg/kg/day; Females: <658 mg/ kg/day) LOAEL
= Males: 555 mg/kg/day; Females: 658 mg/kg/day based on
vacuolation of white matter in brain, increased liver weights,
histopathology in liver...
Eight special mechanistic studies to assess the
CNS white matter vacuolation: White matter vacuolation in the
CNS of mice, rats, and dogs was found to be due to Impurity-5.
Ref: Federal Register. September 7, 2001.
Fluazinam; Pesticide Tolerance. Final Rule.
http://www.fluoridealert.org/pesticides/fluazinam.fr.sept.7.2001.htm
A second oncogenicity study in mice was conducted at 1,000,
3,000 and 7,000 ppm to ensure that an maximum tolerance dose (MTD)
was studied. Findings included increased female mortality, reduced
body weight gains, increased brain weights
and/or liver weights. An impurity in the test material used in
this study resulted in vacuolation of the
white matter of the brain and cervical spinal cord in treated
animals. A statistically significant higher incidence of hepatocellular
adenomas was observed in the 3,000 ppm dose males. Hepatocellular
adenomas are common tumors in male mice. There was no dose relationship
in the induction of the adenoma and no increase in hepatocellular
carcinomas. It was concluded that fluazinam is not carcinogenic
in the mouse.
Ref: Federal Register: December 6, 2000
[Page 76253-76258].
http://www.fluoridealert.org/pesticides/fluazinam.fr.december.2000.htm
Of particular concern
was a neurotoxic lesion described as vacuolation
of the white matter of the brain and sometimes cervical
spinal cord which was initially observed in long-term (1-2 year)
chronic studies in mice and dogs and later, upon careful re-examination
of the central nervous system (CNS), also in shorter-term (4-week
to 90-day) subchronic studies. Although this lesion was also observed
in control animals, the increased incidence and/or severity of
the lesion in test animals was clearly treatment-related and dose-related.
Further investigation of this finding in a series of special studies
demonstrated the same lesion could also be induced in rats. In
the special studies, the following were also determined.
• Fluazinam, per se, was not responsible for the induction
of this lesion. Evaluation of the effects of impurities present
in technical grade fluazinam revealed that one single impurity,
Impurity-5, was solely responsible for the induction of this lesion.
• No significant differences in susceptibility or in incidence
or severity of vacuolation of the white matter of the CNS were
observed between species (mice, dogs, or rats). Similarly, no
significant differences were attributed to sex.
• Electron microscopy of the white
matter (cerebellum) of mice treated with technical grade fluazinam
indicated that treatment-related effects were confined to the
myelin sheaths. Large vacuoles were observed in the intramyelin
sheaths due to the accumulation of fluid between the sheaths.
The nucleus and mitochondria in the oligodendroglia were observed
to remain intact, suggesting no damage to these cells.
• White matter vacuolation in the CNS was reversible. The
myelin sheaths appeared to recover completely during a recovery
period of up to 56 days.
• There appears to be a non-linear dose-response with a clear
threshold below which no effect occurs. It was concluded that
a LOAEL of 0.1 mg/kg/day and a NOAEL of 0.02 mg/kg/day for CNS
effects could be established for Impurity-5.
-- At the current maximum concentration of Impurity-5 in technical
grade fluazinam of 0.1%, the NOAEL for CNS effects of 0.02 mg/kg/day
for Impurity-5 is equivalent to a NOAEL for CNS effects of 20
mg/kg/day for technical grade fluazinam. The NOAEL of 20 mg/kg/day
for CNS effects for technical grade fluazinam is comparable to
the NOAEL for chronic effects for technical grade fluazinam of
1.1 mg/kg/day used to establish the chronic RfD for fluazinam.
Therefore, based on a consideration of all the available data
and information relating to this treatment-related neurotoxic
lesion, it was concluded that the chronic dietary RfD of 0.011
mg/kg/day for “all populations”, including infants and children,
is protective of the CNS effects caused by the presence of Impurity-5
in technical-grade fluazinam.
Ref: US EPA Pesticide Fact Sheet. Fluazinam.
August 10, 2001. http://www.epa.gov/opprd001/factsheets/fluazinam.pdf
SPECIAL/IMPURITIES
-- Comparative— susceptibility to neurotoxicity.
97.0% Impurity-5, 2.0 mg/kg/d, 5
Crj:CD (ICR) mice, 5 Crj:CD (SD) rats, 3 beagles, all male 9 Spontaneous
motor activity in rats and mice. 9 Mean body weight in mice and
rats. 8 Brain weights in mice and rats.
Vacuolation of white matter and swelling of the brain in all treated
animals. Dogs appear to be slightly less susceptible.
-- Comparative— brain sensitivity rats and
mice. 99.5% Impurity-5, 0,
0.5 mg/kg, 5 Crj:CD-1 mice, 5 Crj:CD(SD)
SPF/VAF rats, all female. Incidence
and severity of white matter vacuolation in brains of mice and
rats were similar. Brain weights were comparable to controls.
-- Comparative— brain sensitivity 3- and
10-week old rats and mice—14 days. 99.5%
Impurity-5, 0, 0.5 mg/kg/d, 10 Crj:CD-1 (ICR) SPF/VAF mice
and Crj:CD(SD) SPF/VAF rats,
all male, half 3 weeks old, the other half 10 weeks old
Edema in brains of 1 rat and 2 mice (10
weeks old). Vacuolation in white matter of brains similar between
species of same age, but more severe in older animals compared
to younger.
-- Effect on brain/optic nerves 99.5%.
Impurity-5, 2.5
mg/kg, Crj:CD-1(ICR) mice, 5 males/group, aged 3, 5, 8, 10, 12,
16, 20, 24 weeks All treated mice except
3 week olds had vacuolation of the optic nerve. The effect
was less severe than in the brain. The eyes themselves were normal.
Ages 3, 5, 8 weeks. Trace vacuolation of
white matter in brains Ages 10, 12, 16, 20, 24
weeks Trace to minimal vacuolation of white matter in brains.
-- Various impurities— effect on brains.
96–100% Impurities 1 through 9, doses
correspond to 5000 mg/kg dose of technical, 5 male Crj:CD-1 mice
(Impurity-5 was dosed at 5 mg/kg) Only Impurity-5 was toxic. Coarse
fur, paralysis of hind legs, staggering gait, sedation, moribund.
9 Mean body weight. 8 Brain weight, edema.
Vacuolation of white matter.
-- Recommended
ADI.
0.0011 mg/kg bw/d based on 2 year carcinogenicity in mice (1.1
mg/kg/d with 100- fold UF, three-fold SF for endocrine-related
effects (testicular atrophy, pancreatic exocrine atrophy), and
three-fold for lack of DNT). MOS for other critical endpoint(s)
White matter vacuolation/Neurotox NOEL for white matter vacuolation
was 10 mg/kg/d in chronic dog study (equivalent to 0.02 mg/kg/d
Impurity-5). MOS = 9100. Tumours NOEL for tumours was 3.8
mg/kg bw/d in 2-year rat study. MOS = 3450. Developmental effects
NOAEL for developmental effects was 7 mg/kg bw/d in developmental
rabbit study. MOS = 6350
[ MOS = Margin of Safety
]
Ref:
Canada: Regulatory
Note REG2003-12. Fluazinam. Pest
Management Regulatory Agency. Health Canada. Ottawa. October 27,
2003.
http://www.fluorideaction.org/pesticides/fluazinam.canada.report2003.pd
Fluazuron
- Acaricide - CAS No. 86811-58-7
Note: As there is little data available
on Fluazuron, we include this.
Cattle
Oral application of 2.0 mg/kg b.w. of fluazuron gives rise to
more rapid absorption and maintains higher level of fluazuron
in the bloodstream of cattle than a dermal treatment at the same
dose level (Bull and Strong, 1994). The
compound distributed to the tissues such as muscle, kidney,
liver, lung and brain but deposited
preferentially in the fat. When the radiolabelled fluazuron was
administered subcutaneously to cattles at 1.5 mg/kg b.w., the
mean maximum plasma level of total radioactivity was reached in
48 hours post dose. The radioactivity was absorbed slowly from
the site of injection. Half life of the radioactivity in the blood
was around 78 days (Cameron, B.D., Somers, K. and Speirs, G.C.
(1992). The Distribution and Excretion of [U-14C]Cl-Phenyl CGA
157419 after Subcutaneous Injection to Cattle. Unpublished report
No. 141232. Inveresk Research International Limited, Tranent,
Scotland. Submitted to JECFA by Ciba-Geigy Limited, Basle, Switzerland.)
Ref: Fluazuron. UN Food and Agriculture
Organization. First draft prepared by Dr. P. S. Tantiyaswasdikul,
Department of Pharmacology, Faculty of Pharmaceutical Sciences
Chulalongkorn University, Bangkok, Thailand.
http://www.fao.org/docrep/w8338e/w8338e09.htm#fluazuron
Fluchloralin
- Herbicide
- CAS No. 33245-39-5
Abstract. Basalin,
a formulation of fluchloralin (N-(2-chloroethyl)-2-6-dinitro-N-propyl-4-(trifluoromethyl)-aniline),
is being very widely used as herbicide in large number of important
crops. But very limited data on its toxicological profile is available.
Its adverse effects on central nervous system and locomotor alterations
in sheep given 5 mg/kg orally have been reported. Preliminary
studies in our laboratory indicated alteration in gait in chicken
given Basalin orally @ 200-500 mg/kg followed by ataxia at higher
doses. Since chicken is a suitable model for neurotoxicity assay,
present studies were conducted on one week old broiler chicks
given Basalin daily for four weeks through feed at different dose
levels of 50 mg/kg (Gr I), 100 mg/kg (Gr II) and 150 mg/kg (Gr
III). Each group contained ten chicks. The control chicks (Gr
C) were given equal amount of normal feed. Activities of brain,
liver and plasma acetylcholinesterase (ACHE), carboxylesterase
(CE) and brain neurotoxicesterase (NTE) were estimated, the tissue
esterases after four weeks treatment and plasma esterases at weekly
intervals. The locomoter activity was determined using inclined
plane at alternate days. Histopathological examinations of brains
and spinal cords of four birds from each group were conducted
after four weeks treatment. The data on
all these experiments indicated inhibition of all tissue and plasma
esterases in a dose dependent manner, which were significant in
chicks of Gr III receiving maximum dose for four weeks.
(Brain NTE 70%, brain and liver ACHE 85.71 and
85.45% respectively and liver CE 85.58% of control activity).
The plasma ACHE and CE activities were significantly inhibited
in this group after two weeks onwards (ACHE90.8-90.3%, CE 84.9-84.5%
of control). Alteration in gait in Gr III chicks was observed
after three weeks treatment and was correlated with NTE inhibition.
Histopathological examinations of brain
and spinal cords of chicks receiving maximum dose revealed increased
number of Schwann cells in brain and small numbers of myelinated
nerve fibers in spinal cord. The studies thus indicated
possibility of neuropathic effects of Basalin on prolonged exposure
or at higher doses.
Ref:
Basalin induced neurotoxic effects in broiler chicks; by Sushma
Rishi and Uma Arora. Toxicology Letters; Volume 95, Supplement
1 , July 1998, Pages 144-145.
Flucythrinate
-
Acaricide, Insecticide - CAS No. 70124-77-5
-- Groups of 50 male
and 50 female CD-1 mice received technical flucythrinate (80%
pure) in the diet at 0, 30, 60, or 120 ppm daily for 18 months.
Skin lesions (abrasions, ulceration and scabs) were observed in
high-dose males and females. No treatment-related symptoms or
treatment-related changes in survival were found. No haematology,
clinical chemistry or urinalysis were undertaken. At necropsy,
hepatocellular adenomas were found in all
control and treated groups. The incidence was variable and
statistically- significant only in high-dose males. Hepatocellular
adenocarcinoma and hepatocellular carcinoma were found in low
incidence in all male groups, but only in control and low-dose
female mice. The incidences of these neoplasms were similar to
those previously found in mice and were apparently unrelated to
treatment. Mild sciatic nerve degeneration occurred in all groups,
but at slightly increased incidence in treated groups, especially
high-dose males. There was no apparent dose-response relationship.
The incidence of mild axonal degeneration
was similar in all groups. The no-effect level for this
study was therefore set at 30 ppm (Lang, 1981b).
Ref: 1985 World Health Organization Review
for Flucythrinate.
http://www.fluoridealert.org/pesticides/flucythrinate.1985.who.htm
•
Note from FAN:
--
axonal degeneration is associated with Multiple Sclerosis
see http://www.albany.net/~tjc/abstr05a-2g1.html#1
-- "Interestingly, although about 30% of individuals with
HIV/AIDS will develop the symptoms of sensory neuropathy, axonal
degeneration is almost universal at autopsy."
Ref: Griffin, et al., Peripheral neuropathy
in AIDS: New Investigative Approaches. Technical advances in AIDS.
In: Major E, ed. Research in Human Nervous System. Plenum Press:
1994.
see - http://www.hopkins-aids.edu/publications/report/may01_2.html
]
Flufenacet
- Herbicide - CAS No. 142459-58-3
NOEL = 40 ppm [1.29
mg/kg/day in males and 1.14 mg/kg/day in females] LOEL = 800 ppm
[27.75 mg/kg/day in males and 26.82 mg/kg/day in females] based
on increased alkaline phosphatase, kidney, and liver weight in
both sexes, increased cholesterol in males, decreased T3, T4 and
ALT values in both sexes, and increased incidence of microscopic
lesions in the brain [axonal degeneration],
eye [vacuolization of the ciliary body epithelium], kidney [hyperplasia
of the epithelial cells], spinal cord [axonal degeneration], sciatic
nerve [axonal degeneration] and liver [hepatocytomegaly].
Ref: EPA Pesticide Fact Sheet, April 1998.
http://www.epa.gov/opprd001/factsheets/flufenacet.pdf
•
Note from FAN:
--
axonal degeneration is associated with Multiple Sclerosis
see
http://www.albany.net/~tjc/abstr05a-2g1.html#1
-- "Interestingly, although about 30% of individuals with
HIV/AIDS will develop the symptoms of sensory neuropathy, axonal
degeneration is almost universal at autopsy."
Ref: Griffin, et al., Peripheral neuropathy
in AIDS: New Investigative Approaches. Technical advances in AIDS.
In: Major E, ed. Research in Human Nervous System. Plenum Press:
1994.
see - http://www.hopkins-aids.edu/publications/report/may01_2.html
-- Chronic toxicity:
A 1-year dog chronic feeding study
with a NOAEL was 40 ppm (1.29 mg/kg/day in males and 1.14 mg/kg/day
in females), and a LOAEL of 800 ppm (27.75 mg/kg/day in males
and 26.82 mg/kg/day in females) based on increased alkaline phosphatase,
kidney, and liver weight in both sexes, increased cholesterol
in males, decreased T2, T4 and ALT values in both sexes, and increased
incidences of microscopic lesions in the
brain, eye, kidney, spinal cord, sciatic nerve, and liver.
-- Metabolite toxicology. A 55-day
dog study with subcutaneous administration of thiadone
flufenacet metabolite supports the hypothesis that limitations
in glutathione interdependent pathways and antioxidant stress
result in metabolic lesions in the brain
and heart following flufenacet exposure.
-- A rat subchronic neurotoxicity study with a NOAEL of 120 ppm
(7.3 mg/kg/day in males and 8.4 mg/kg/day in females), and a LOAEL
of 600 ppm (38.1 mg/kg/day in males and 42.6 mg/kg/day in females)
based on microscopic lesions in the cerebellum/medulla
and spinal cords.
Ref:
Federal Register. March 29, 2000. Notice of Filing a Pesticide
Petition to Establish a Tolerance for Certain Pesticide Chemicals
in or on Food.
http://www.fluoridealert.org/pesticides/flufenacet.fr.mar.29.2000.htm
Chronic feeding studies
in dog and rat showed structural or functional alterations in
liver, kidney, haematology, spleen, and
thyroid. Flufenacet induces neuropathogical changes in the brain
and spinal cord (axonal swelling)
in rat and dog. The overall evaluation of the observed
changes demonstrates that these effects occur only after repeated
and prolonged exposure to high dose levels of flufenacet, which
saturate metabolic pathways, and exceed the animal capacity to
rapidly metabolise and excrete it. The liver was considered the
primary target organ, with increases in organ weight, cell size
and number, and/or associated changes in liver function tests.
Ref: European Commission, Health & Consumer
Protection Directorate-General, Scientific Committee on Plants,
October 17, 2001. SCP/FLUFEN/002-Final.
http://europa.eu.int/comm/food/fs/sc/scp/out112_ppp_en.pdf
Special Studies: In
a 55-day dog study subcutaneous via mini-pump with Thiadone [flufenacet
metabolite] support the hypothesis that
limitations in glutathione interdependent pathways and antioxidant
stress result in metabolic lesions in the brain and heart
following flufenacet exposure.
Ref:
US EPA. Pesticide Fact Sheet. Flufenacet Reason for Issuance:
Conditional Registration Date Issued: April 1998.
http://www.epa.gov/opprd001/factsheets/flufenacet.pdf
Flumioxazin
- Herbicide - CAS No. 103361-09-7
-- ONCOGENICITY, MOUSE
** 050; 184618; "Oncogenicity Study of S-53482 by Dietary
Administration in Mice"; (T. Seki; Environmental Health Science
Laboratory, Sumitomo Chemical Co., Ltd., Osaka, Japan; Project
ID 1928; 9/24/93); Fifty one Crj:CD-1 (ICR) mice/sex/group were
treated in the diet with 0, 300, 3000 or 7000 ppm of S-53482 (lot
no. PYG-89021-M, purity: 94.8%) for 78 weeks ((M): 0, 31.1, 314.9,
754.1 mg/kg/day, (F) 0, 36.6, 346.4, 859.1 mg/kg/day). An additional
15 animals/sex/group were treated for 52 weeks (satellite)...
An increased incidence of calcification
in the brain was noted for the 3000 and 7000 ppm males
which survived to the conclusion of the study (0: 3/37 vs. 3000:
11/36 (p<0.05), 7000: 13/38 (p<0.01).
-- "The Pharmacokinetics of [ 14 C]S-53482 in the Rat 1 ,
Biliary Excretion of [ 14 C]S-53482 in the Rat 2 , Tissue Distribution
of [ 14 C]S-53482 in the Rat 3 "; (Gibson, N.A., G.R. Krautter,
K. Jalali, and L.O. Ruzo; PTRL East, Inc., Richmond, CA and PTRL
West, Inc., Richmond, CA; Project IDs: 1034E 1 , 1035E/588W 2
, 1036E/589W 3 ; 2/19/97 1 , 3/6/97 2 , 3/17/97 3 ); In the pharmacokinetic
study, 7 Sprague-Dawley (Crl:CD:BR) rats/sex were dosed by oral
gavage with 1 or 100 mg/kg of [Tetrahydrophthaloyl-1,2-14 C]S-53482
(Flumioxazin Technical) (lot no. RIS96014, specific activity:
121 mCi/mmole, radiochemical purity: 98.9%, chemical purity: 99.0%)
mixed with; unlabeled S-53482 (lot no. 60208AG, purity: 99.9%)...
Only a minimal amount of the radiolabel appeared to penetrate
the blood-brain barrier.
Ref:
January 2003 (revised) -
Summary of Toxicological
Data. California EPA, Department of
Pesticides Regulation, Medical Toxicology Branch.
--
Rats. A 90-day subchronic toxicity study was conducted in rats,
with dietary intake levels of 0, 30, 300, 1,000 and 3,000 ppm
flumioxazin technical (98.4% purity). The NOAEL of 300 ppm was
based on decreased bwts; anemia; increases
in absolute and/or relative liver, kidney, brain,
heart, and thyroid weights, and histological changes in the spleen,
liver, and bone marrow related to the anemia.
Ref: Federal Register: February 14, 2001
[Notices] [Page 10292-10301]. Notice of Filing a Pesticide Petition
to Establish a Tolerance for a Certain Pesticide Chemical in or
on Food.
http://www.fluoridealert.org/pesticides/flumioxazin.fr.feb.14.2001.htm
Fluometuron
- Herbicide - CAS No. 2164-17-2
-- Organ Toxicity.
Toxic injury to the liver,
kidneys, gut and brain is induced
when lethal doses of fluometuron are administered experimentally
(TOXNET. 1986. National library of medicine's toxicology data
network. Hazardous Substances Databank. Public Health Service.
National Institute of Health, U. S. Department of Health and Human
Services. Bethesda, MD: NLM.).
Fluoroacetamine
-
Insecticide, Rodenticide - CAS No. 640-19-7
(also known as
Fluoroacetamide or Compound 1081)
Health Hazards (Acute,
Delayed, and Chronic): This material is super toxic; probable
oral lethal dose in humans is less than 5 mg/kg, or a taste (less
than 7 drops) for a 150-lb. person (*Gosselin 1976). Chemically
inhibits oxygen metabolism by cells with critical
damage occurring to the heart, brain,
and lungs resulting in heart failure, respiratory arrest, convulsions,
and death (Gilman 1980, p. 1644).
Ref: US EPA Chemical Profile. October 31,
1985
http://www.fluoridealert.org/pesticides/fluoroacetamide.epafacts.87.htm
Neurologic sequelae
have been noted following acute poisoning, including hypertonicity
with arm and leg spasms, severe mental deficits, and moderate
residual paresis. Severe cerebellar dysfunction,
ataxia, and depression were described
in a 15-year-old patient who survived acute fluoroacetate poisoning.
Ref:
FLUOROACETAMIDE CASRN: 640-19-7. Hazardous Substances Data Bank.
http://www.fluorideaction.org/pesticides/fluoroacetamide.hsdb.htm
Sodium fluoroacetate
and fluoroacetamide are readily absorbed by the gut, but only
to a limited extent across skin. The toxic mechanism is distinct
from that of fluoride salts. Three molecules of fluoroacetate
or fluoroacetamide are combined in the liver to form a molecule
of fluorocitrate, which poisons critical enzymes of the tricarboxylic
acid (Krebs) cycle, blocking cellular respiration. The heart,
brain, and kidneys are
the organs most prominently affected... Crimidine and sodium
fluoroacetate are no longer registered for use as pesticides.
Ref: US EPA.
http://www.epa.gov/oppfead1/safety/healthcare/handbook/Chap17.pdf
Fluoroacetic
Acid - Rodenticide -
CAS No. 144-49-0
Little specific data
were available specifically about the toxicity of fluoroacetic
acid; its toxicity is expected to be similar to that of FLUOROACETATE.
-- FLUOROACETIC ACID WAS FOUND IN BRAIN
TISSUES FOR ONLY 1 MIN AFTER INJECTION EVEN THEN IT WAS
LESS THAN 15% OF AMT INJECTED. [MORSELLI PL ET AL; BIOCHEM PHARMACOL
17 (2): 195 (1968)]
-- Neurologic sequelae have been noted following acute poisoning,
such as hypertonicity with arm and leg spasms, severe mental deficits,
and moderate residual paresis. Severe cerebellar
dysfunction, ataxia, and depression were described in a
15-year-old patient who survived acute fluoroacetate poisoning.
-- FLUOROACETIC ACID HAS BEEN SHOWN TO BE CONVERTED IN VIVO INTO
FLUOROCITRATE. [Fluorocitrate is a glial
toxin -- see http://ethesis.helsinki.fi/julkaisut/laa/biola/vk/reenila/results.html)
].
-- FLUOROACETIC ACID WAS FOUND IN BRAIN
TISSUES FOR ONLY 1 MIN AFTER INJECTION EVEN THEN IT WAS
LESS THAN 15% OF AMT INJECTED.
[MORSELLI PL ET AL; BIOCHEM PHARMACOL 17 (2): 195 (1968)]
Ref: TOXNET profile from Hazardous Substances
Data Bank.
http://www.fluoridealert.org/pesticides/fluoroacetic.acid.toxnet.htm
Fluorouracil
-
Former insect Chemosterilant; now used as a pharmaceutical -
CAS No. 51-21-8
Chronic neurotoxic
effects were noted in dogs fed fluorouracil at a dietary dose
of 2 mg/kg/day for 6 months. In this study, animals were examined
at the end of 3 months and 6 months. At the end of the experiment,
or at death, the brain was removed and examined (only one dog
survived the entire 6-month period). Histological
sections of the brain showed the presence large multiple monolocular
vacuoles in the wall of the fornix of the third ventricle.
Ref: USEPA/OPPT. Support Document
for the Health and Ecological Toxicity Review of TRI Expansion
Chemicals. U. S. Environmental Protection Agency, Washington,
DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV.
40 CFR Part 372. Addition of Certain Chemicals; Toxic Chemical
Release Reporting; Community Right-to-Know; Proposed Rule.
-- Absorption, Distribution
& Excretion : Fluorouracil is distributed into tumors, intestinal
mucosa, bone marrow, liver, and other tissues. Despite its limited
lipid solubility, the drug readily crosses
the blood-brain barrier and distributes
into CSF and brain tissue... [McEvoy, G.K. (ed.). American
Hospital Formulary Service - Drug Information 93. Bethesda, MD:
American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements,
1993).
-- Potential Adverse Effects on Fetus: Exposure in first trimester:
skeletal abnormalities; hypoplasia of aorta, lungs, thymus, and
gastrointestinal tract; and urinary tract abnormalities. Fetus
exposed in third trimester had cyanosis and clonus... the incidence
of malformations, particularly those affecting the tail,
hindlimb bud, and brain, was increased.
Ref: TOXNET profile from Hazardous Substances
Data Base.
http://www.fluoridealert.org/pesticides/fluorouracil.toxnet.hsdb.htm
-- PubMed
abstract: Two metabolites of 5-fluorouracil
(FU), monofluoroacetic acid (FA) and alpha-fluoro-beta-alanine
(FBAL), were continuously administered into the left ventricle
of the brain in cats for up to 1 month to investigate the mechanism
of neurotoxicity of FU and its derivatives. The cumulative doses
of FU and FBAL over a 1-month period were 1.5-45 mg (20 cats)
and 0.2-4.8 mg (21 cats), respectively. As controls for each experimental
group, acetic acid (AA) and beta-alanine (BAL) were administered.
In terms of survival time in relation to the cumulative dose and
molecular weight, FBAL was more toxic than FA. Neuropathologically,
two types of change, vacuoles and
necrosis/softening-like change, were found.
The vacuoles
were 20-50 microns in diameter, and distributed mainly in the
cerebellar nuclei, white matter and the tectum and tegmentum of
the brain stem in both experimental groups. Electron microscopically,
these vacuoles were due to splitting of the myelin intraperiod
line or separation between the axon and the innermost layer of
myelin. Necrosis/softening-like change occurred preferentially
in the FBAL group and was located symmetrically in the superior
and inferior colliculi, oculomotor nuclei and thalamus.
Both types of neuropathological change, especially those in the
FBAL group, were similar to those found in cats orally administered
with FU and its derivatives.(ABSTRACT TRUNCATED AT 250 WORDS)
Ref: Acta Neuropathol (Berl) 1990;81(1):66-73;
Experimental
neurotoxicity of 5-fluorouracil and its derivatives is due
to poisoning by the monofluorinated organic metabolites, monofluoroacetic
acid and alpha-fluoro-beta-alanine; Okeda R, Shibutani
M, Matsuo T, Kuroiwa T, Shimokawa R, Tajima T.
Fluoride
Non-Human
Toxicity Excerpts : /ACUTE POISONING/ IF SUFFICIENT FLUORIDE
IS ABSORBED ... FLUORIDE ION INCREASES CAPILLARY PERMEABILITY
AND ALSO PRODUCES A COAGULATION DEFECT. THESE ACTIONS LEAD
TO HEMORRHAGIC GASTROENTERITIS & HEMORRHAGES, CONGESTION,
& EDEMA IN VARIOUS ORGANS INCL THE BRAIN.
CLINICAL MANIFESTATIONS ... INCLUDE EXCITABILITY, MUSCLE
TREMORS, WEAKNESS, URINATION, DEFECATION, SALIVATION, EMESIS,
SUDDEN COLLAPSE, CLONIC CONVULSIONS, COMA, & DEATH DUE TO
RESP & CARDIAC FAILURE. CYANOSIS & EARLY RIGOR MORTIS ...
. /FLUORIDE/
Ref: Booth, N.H., L.E. McDonald (eds.).
Veterinary Pharmacology and Therapeutics. 5th ed. Ames,
Iowa: Iowa State University Press, 1982. 1014
Website search for "Ammonium silicofluoride" (CAS
No. 16919-19-0) at:
http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
|
Flurtamone
- Herbicide - CAS No. 96525-23-4
-- Two-year dietary
study in mice. In a carcinogenicity study, groups CD-1 mice (60/sex/dose)
were fed diets containing 0, 30, 300, 3500 and 7000 ppm flurtamone
(91.9% purity) for at least 78 weeks... The mean intake of flurtamone
for males and females was estimated to be 4.5, 45, 525 and 1050
mg/kg bwday at 30, 300, 3500 and 7000 ppm, respectively. After
78 weeks of treatment, the mortality rate for the 7000 ppm female
mice was significantly higher than controls and a significant
negative survival trend was found in both sexes. The test laboratory
pathologist considered systemic amyloidosis
to be the major cause of death (Table 5.34) [page 102]... Mean
relative brain weights were significantly increased in
F0 females at dose-levels ≥2000 ppm, and in F1 males and females
at 5000 ppm. While mean absolute brain weights
were significantly reduced in F1 males and females at dose
levels ≥2000 ppm. Other small but statistically significant changes
in mean organ weight were considered to be incidental, related
to body weight effects or lacked a dose-response relationship.
No macroscopic changes were observed which could be related to
treatment. The only microscopic finding which could be attributed
to compound administration was centrilobular hypertrophy of the
liver. Dose-related increases in the incidence of centrilobular
hypertrophy were observed in F0 and F1 animals at dose levels
≥500 ppm (Table 5.31). this centrilobular hypertrophy was considered
to be an adaptive biological response [page 108].
Ref: December
2000 . Evaluation on: Flurtamone.
No. 196. Department for Environment, Food and Rural Affairs, Pesticides
Safety Directorate, Mallard House, Kings Pool, 3 Peasholme Green,
York YO1 7PX, UK.
Available online at:
http://www.pesticides.gov.uk/citizen/evaluations/evallist_alphabet.htm
Flusilazole
- Fungicide - CAS No. 85509-19-9
Abstract: Triazole-derivatives alter the pharyngeal apparatus
morphogenesis of rodent embryos cultured in vitro. The hindbrain
segmentation and the rhombencephalic neural crest cell (NCCs)
migration are altered by Fluconazole exposure in vitro. The aim
of the present work is to identify if a common pathogenic pathway
is detectable also for other molecules of this class of compounds.
9.5 days post coitum (d.p.c.) old rat embryos were exposed in
vitro to the teratogenic concentrations of Flusilazole, Triadimefon
and Triadimenol and cultured for 24, 48 or 60 h. The expression
and localisation of Hox-b1 and Krox-20 proteins (used as markers
for hindbrain segmentation) were evaluated after 24 h of culture.
The localisation and distribution of NCC was evaluated after 24,
30 and 48 h of culture. The morphology of the embryos was analysed
after 48 h, while the branchial nerve structures were evaluated
after 60 h of culture. Hindbrain segmentation and NCC migration
alteration as well as pharyngeal arch and cranial nerve abnormalities
were detected after exposure of the tested molecules. A
common severe teratogenic intrinsic property for the tested molecules
of this chemical class has been found, acting through alteration
of the normal hindbrain developmental pattern.
Reference: Menegola E, Broccia ML, Di Renzo
F, Massa V, Giavini E (2005). Study on the common teratogenic
pathway elicited by the fungicides triazole-derivatives. Toxicol
In Vitro. Sep;19(6):737-48.
Abstract available at:
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15913947&query_hl=19
1,2,4-triazole targets the nervous
system, both central and peripheral, as
brain lesions (most notably in the cerebellum)
were seen in both rats and mice, and peripheral nerve degeneration
was also seen in the subchronic neurotoxicity study in rats. In
addition, brain weight decreases were
seen in several studies, including in the offspring in the reproductive
toxicity study. In the subchronic/neurotoxicity study, there
is evidence that effects progress over time, with an increase
in incidence of clinical signs (including tremors and muscle fasciculations)
during weeks 8 and 13 that were not seen during earlier evaluations.
Effects were also seen on reproductive organs in both sexes, most
notably ovaries (in rats) and testes (in rats and mice), in both
the reproductive toxicity and subchronic toxicity studies. Hematological
changes, including slightly decreased hemoglobin and/or hematocrit,
have also been seen in multiple studies and species (in rats at
doses of 33 mg/kg/day and above, and in mice at doses of 487 mg/kg/day
and above). Studies depicting the effects of chronic exposure
to free triazole or its conjugates are not currently available.
Ref: Human
Health Aggregate Risk Assessment for Triazole-derivative Fungicide
Compounds (1,2,4-Triazole, Triazole Alanine, Triazole Acetic
Acid). US EPA, February 7, 2006.
abnormalities at the level of the branchial apparatus; disorganisation
and fusions at the level of the cranial nerves; abnormalities
in the migration of NCC, not able to form 3 distinct migration
stripes from the rhomboencephalon to the branchial apparatus;
alteration of the hindbrain segmentation, with reduced and scattered
immunolocalised stripes.
Reference: Massa et al. Mechanisms Involved
In Triazole-Induced Teratogenesis: In Vitro Study. Toxicol Lett
2003 Sep ;144 (Suppl 1 ):S107
Flutolanil
- Fungicide - CAS No.
66332-96-5
Chronic toxicity. A
1-year dog chronic feeding study with a NOEL was 40 ppm [1.29
mg/kg/day in males and 1.14 mg/kg/day in females] and a LOEL of
800 ppm [27.75 mg/kg/day in males and 26.82 mg/kg/day in females]
based on increased alkaline phosphatase, kidney, and liver weight
in both sexes, increased cholesterol in males, decreased T2, T4
and ALT values in both sexes, and increased
incidences of microscopic lesions in the brain,
eye,
kidney, spinal cord, sciatic nerve and liver.
Federal Register: June 23, 1998 [Page 34176-34184].
Notice of Filing of Pesticide Petitions.
http://www.fluoridealert.org/pesticides/Flutolanil.FR.June.23.1998.htm
Fluvalinate
- Acaricide, Insecticide - CAS No. 69409-94-5
Eight days after a
pregnant cow was given a single a single dose of 14 C-fluvalinate,
only trace amounts (approximately 1x10 -5 % of the administered
dose) of 14 C were detected in the fetus (Quistad et al. 1982).
However, given the fact that exposure of rat fetuses to pyrethroids
via their mothers resulted in persistent alterations in brain
neurotransmitter numbers (Malaviya et al. 1993), it would
appear that concentrations that reached the fetal brain were sufficient
to cause a consistent effect. (p 94-95)
Ref: September 2001. Draft Toxicological
Profile for Pyrethrins and Pyrethroids. U.S. Department of Health
and Human Services. Public Health Service. Agency for Toxic Substances
and Disease Registry.
http://www.atsdr.cdc.gov/toxprofiles/tp155.pdf
Fluvalinate, at dose
rates of 10.5 and 21 mg/kg (1/10th and 1/5th of LD50, respectively)
by ip route in rats, impaired acquisition
(learning), while no such effect on permanent memory was
observed. Consolidation process of memory
traces (temporary memory) was impaired at lower doses whereas
it was completely suppressed at higher dose. However, at both
the dose levels retrieval of passive avoidance reaction was completely
suppressed. [Maity NK, Punia JS; Indian J Exp Biol 29 (2):
178-9 (1991)]
Ref: Hazardous Substances Data Bank for
FLUVALINATE CASRN: 69409-94-5.
http://www.fluorideaction.org/pesticides/fluvalinate.toxnet.hsdb.htm
|

