|
Return to
Adverse Effects
Abstracts
ACTIVITY:
Fungicide
(azole)
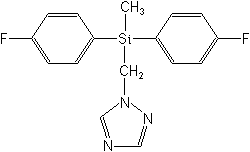
CAS Name:
1-[[bis(4-fluorophenyl)methylsilyl]methyl]-1H-1,2,4-triazole
Structure:
| Adverse
Effects:
Bladder
Blood
Body Weight Decrease
Bone - Cleft palate
Brain
Conjoined Twins
Developmental
Endocrine: Suspected
Disruptor
Endocrine: Testicular
Eye - Microphthalmia and Coloboma
Kidney
Liver
Reproductive
Teratogenic
Tongue |
| Environmental
Effects:
•
Reproductive
process in
fish
• Persistent in soil
• Potential for impact on reproductive process of fish
•
Acute toxicity to oysters - shell deposition
• Acute toxicity to mysid shrimp
• Acute toxicity to sheepshead minnow |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
No
- Revoked
•• Pending for emergency exemption
use as of September 2005 (see Federal
Register below) |
| US
EPA PC Code: |
128835 |
| California
Chemical Code |
2278 |
| FDA
LMS Code: |
950 |
Registered
use in
(includes only a limited list of countries)
|
Argentina,
Australia, Canada, Germany, Hungary, Japan, Korea, New Zealand,
Portugal, South Africa, UK
Australia:
Grape |
| Canada's
Maximum Residue Levels (MRLs) |
As
of Sept 13, 2000:
Apples, Milk, Meat and Meat byproducts
Imported
bananas, grapes (0.5 ppm) and raisins |
| Japan's
Maximum Residue Levels (MRLs) |
Apple,
Apricot, Banana, Barley, Cherry, Grape, Loquat, Nectarine,
Other cereal grains, Other oil seeds, Peach, Japanese pear,
Pear, Mume Plum, Quince, Rape seeds, Rye, Sugar
beet, Sugarcane, Sunflower seeds, Wheat |
| Other
Information |
| Molecular
Formula: |
C16H15
F2 N3Si |
| Entry
Year: |
1985 |
| Inventing
Company: |
DuPont |
| Other
Names: |
DPX-H6573,
Nustar,
Olymp,
Punch |
| Manufacture
site: |
US:
DuPont, La Porte Texas 77571 |
| Of
special interest: |
| PAN
Data |
| Material
Safety Data Sheets & Labels |
October
6, 2005 - Comments
on Flusilazole submitted to US EPA.
Docket No. OPP-2005-0242. Submitted by Fluoride Action Network
Pesticide Project. |
| DuPont
(2005) - Summary of data
compiled in support of a Section 18 Emergency Exemption
request for control of Asian Rust on Soybeans. DuPont Punch
(active ingredient: Flusilazole) and DuPont Charisma (Active
ingredients: Flusilazole and Famoxadone). |
June
8, 2005 - Multi-national giant DuPont
is planning to enter India's infrastructure industry in a big
way besides expanding its agriculture seed and coating and colour
technologies business in the country, its chief executive said
on Wednesday. "India has been our traditional base where
we have been trying to enter the coating technology market in
a big way. Now, the infrastructure sector was opening up and
we would also be keen to register our presence," DuPont
chairman and chief executive officer Chad Holliday told PTI
Nagoya, Japan.... Later briefing visiting reporters, Holliday
said DuPont's market in China was around $1 billion. Referring
to the company's achievements in the agricultural seed sector,
Holliday recalled that Indian farmers were amazed at the results
on utilising Indoxcarb, a branded cotton insecticide. "Similarly
in Argentina we helped farmers fight Asian Soyabean Rust, a
devastating new disease with our best-in-class fungicide Flusilazole,"
company vice-president Uma Chowdhry told PTI. Powell said the
company earned 42 per cent of its revenue from USA, 28 per cent
from Europe and 17 per cent from Asia.
Ref: DuPont eyes India's core sector; by Priyadarshi Siddhanta
in Nagoya | June 08. rediff.com
|
| July
2002 -
Opinion of the Scientific
Committee on Plants on specific questions
from the Commission concerning the evaluation of flusilazole
in the Council Directive 91/414/EEC. European Commission. Health
& Consumer Protection Directorate-General. |
| June
2002 - In Australia when Flusilazole is used
"As a fungicide in quarantine use on mango trees"
no maximum residue levels are required.
Ref: June 2002. Table 5. Uses of substances where maximum
residue limits are not necessary. Australian National Registration
Authority for Agricultural Veterinary Chemicals. The MRL Standard.
Maximum residue limits in food and animal feedstuff. http://www.nra.gov.au/residues/mrl5.pdf
|
| April
29, 2000. UK Department for Environment, Food & Rural Affairs
in the online report, "Design of a Tax or Charge Scheme
for Pesticides. " Annex C3: Overview of Pesticide Industry |
| April
2002 - Beer
in the UK: 23 organofluorine pesticides approved for use on
malting barley. Published by the British
Beer and Pubs Association and Brewing Research International. |
| October
15, 2000 -
Parents
believe that exposure to Flusilazol during pregnacy resulted
in severe eye deformities such as
microphthalmia (small eyes) and coloboma, a defect in the structure
of the eyes. Ref: Scottish Daily Record & Sunday Mail Ltd.
- Sunday Mail. |
| June
1999 -
Used in Benlate. Article
on Lake Apopka [Florida] restoration. |
| 1990's
-
A contaminant found
in another DuPont fungicide: Benlate - Articles
published in the Tampa Tribune, Florida. |
| April
2000 -
Food and Drug Administration
Pesticide Residue Monitoring. - Table
3. Pesticides detectable by methods used in 1999 regulatory
monitoring. |
| One
of 8 fluorinated pesticides used to cultivate grapes in Australia. |
| Abstracts |
| 1993
-
Pesticide residues in food.
FAO/WHO. |
Hazard Characterization (Page
13)
1,2,4-triazole (free triazole)
is a metabolite common to a number of triazole-derivative
pesticides, and is found in both mammalian (rat) and plant
metabolism studies. Although for most
pesticides, mammals convert only a small proportion to free
triazole (less than 25%), two
compounds (tetraconazole
and flusilazole) demonstrate relatively high conversion
(68-77%) in rat metabolism studies.
As a plant metabolite, and given the wide use of triazole-derivative
pesticides (used as fungicides on many crops as well as
on turf) free triazole is found in a variety of food commodities,
including animal byproducts. 1,2,4-triazole appears to be
relatively stable in the environment, and may be found in
rotational crops as well as in water...
Source: Human
Health Aggregate Risk Assessment for Triazole-derivative
Fungicide Compounds (1,2,4-Triazole, Triazole Alanine,
Triazole Acetic Acid). US EPA, February 7, 2006.
|
| Flusilazole
ranked number 10 for "Most Widely
used pesticides in the UK (by Area Treated)" and "Carbendazim/flusilazole"
ranked number 19 in the same category.
| Rank
|
Formulation |
Method |
Area
treated (ha) |
Weight
applied (kg ai) |
| 10 |
Flusilazole |
Spray |
809,648 |
90,564 |
| 19 |
Carbendazim/flusilazole |
Spray |
543,185 |
94,324 |
Ref:
April 29, 2000. UK Department for Environment, Food &
Rural Affairs in the online report, "Design of a Tax
or Charge Scheme for Pesticides. " Annex C3: Overview
of Pesticide Industry
http://www.fluorideaction.org/pesticides/pesticides.ranks.uk.2000.htm
|
| Federal
Register |
| Date |
Docket
No. |
Details |
| September
21, 2005 |
OPP-2005-0242 |
Application
for Emergency Exemption. EPA has received a quarantine
exemption request from the Minnesota and South Dakota Departments
of Agriculture to use the pesticide flusilazole (Punch
3.3EC), CAS No. 85509-19-9, and a flusilazole + famoxadone
premix (Charisma 1.7 EC) on soybeans
to control Asian soybean rust. The Applicant
proposes the use of a new chemical which has not been registered
by the EPA. EPA is soliciting public comment before
making the decision whether or not to grant the exemption.
•
See
•
March
2005 request from the states of Minnesota and South Dakota
(Docket No. OPP-2005-0242-0002)
-- Cyproccnazole,
metconazole, flusilazole, prothioconazole,
and flutriafol are triazole fungicides.
EPA is evaluating the toxicological significance of triazole
metabolites 1,2,4-triazole, triazolylalanine (TA) and triazolylacetic
acid (TAA). Of the three metabolites,
only 1,2,4-triazole can be considered toxicologically significant
and EPA has identified developmental toxicity as the endpoint
of concern. An industry Triazole
Task Force has submitted worst-case studies (Appendix
J, original submission) to EPA demonstrating a reasonable
certainty of no harm for 1,2,4-triazole stemming from triazole
detivative fungicides for food only, as well as for food
and water (p 29).
-- The
manufacturers of flusilazole (Punch) and flusilazole + famoxadone
(Charisma) -DuPont Crop Protection,
Inc.; and, flutriafol (Impact) -Cheminova,
are aware of and supportive of this request. Letters of
support and draft labels for soybeans have been received
(Appendix 3, this addendum) (p 30).
-- This
request is the first request for the use of each of the
requested products; Alto, Quadris Xtra, Caramba,
Headline-Caramba, Operetta, Punch, Charisma, JAU 6476, and
Impact on soybean by all states (p 30).
-- Flusilazole
- DuPont is actively pursuing registration of flusilazole
on soybeans. See Appendix
1. this addendum for studies on product chemistry, toxicology
and ecotoxicology, environmental fate and residues (p 30).
•
Appendix
1: DuPont Punch (active ingredient: Flusizole) and DuPont
Charisma (Active ingredients: Flusilzole and Famoxadone).
Summary of data compiled in support of a Section 18 Emergency
Exemption request for control of Asian Rust on Soybeans.
•
See
•
October 5, 2005, Comments
from the American Soybean Association. Docket No. OPP-2005-0242-0003.
•
October 6, 2005,
Comments
on Flusilazole submitted to US EPA. Docket
No. OPP-2005-0242. Submitted by Fluoride Action Network Pesticide
Project. Docket
No. OPP-2005-0242-0004. |
2002
- Zerulla M, LSnge R, Steger-Hartmann T, Panter G, Hutchinson
T,and Dietrich DR. Morphological sex
reversal upon short-term exposure to endocrine modulators
in juvenile fathead minnow (Pimephales promelas).
Toxicology Letters 131: 51-63.
2001
- Blązquez M, Felip A, Zanuy S, Carrillo M, Piferrer F.
Critical period of androgen-inducible
sex differentiation in a teleost fish, the European sea
bass. Journal of Fish Biology 58: 342-358.
|
SUPPORT:
A DERMAL DOSE RANGE-FINDING PRENATAL DEVELOPMENTAL TOXICITY
STUDY OF FLUSILAZOLE IN RATS, FINAL REPORT, WITH COVER LETTER
DATED 3/6/1998 (SANITIZED)
Corporate
Name: WIL RESEARCH LABORATORIES INC
Source:
EPA/OTS; Doc #89980000145S
Keywords:
CONFIDENTIAL
FLUSILAZOLE
HEALTH EFFECTS
REPRODUCTION/FERTILITY EFFECTS
TERATOGENICITY
MAMMALS
RATS
DERMAL
CAS
Registry Numbers: 85509-19-9
Order Number: NTIS/OTS0559123
Note
from
FAN: if anyone has a copy of this report please send
us a copy. Thanks. EC.
|
1993
FAO/WHO JOINT MEETING ON PESTICIDE RESIDUES Geneva, 20-29 September
1993
PESTICIDE
RESIDUES IN FOOD
REPORT OF THE 1993 JOINT FAO/WHO MEETING OF EXPERTS
4.24
FLUSILAZOLE (165)
RESIDUE
AND ANALYTICAL ASPECTS
Flusilazole
was previously reviewed for residues by the 1989, 1990 and 1991
Meetings. The present Meeting reviewed information provided in
response to the 1991 JMPR requirement for additional GAP and residue
data to confirm the 0.1 mg/kg temporary estimate for peaches and
nectarines, and information listed as desirable on grapes, details
of wheat grain freezer storage studies, stability of metabolites
in freezer-stored grain samples, hen metabolism, metabolites in
grain processed fractions and soil studies. Additional residue
data on pome fruit, grapes and cereals (although there were no
outstanding residue data requirements on these commodities) and
new data on sugar cane were also provided.
Fate of
residues in animals. Several reports on hen metabolism were
provided. Some had been submitted before and some, including the
requested study by Smyser, were new. Only the Smyser report included
data in need of review by the Meeting.
The report
basically combines and summarizes information in two previously
reviewed reports and provides further clarification of the residues
of metabolites, especially in terms of the percentage of the total
radioactivity in poultry tissues and eggs, for both the phenyl
and triazole labels. It confirms previous JMPR conclusions that
bis(4-fluorophenyl)(methyl)silanol (IN-F7321, the methyl silanol)
and 4-fluorophenyl(methyl)silanediol are the predominant residues
in poultry tissues and eggs arising from the phenyl-labelled compound
and that triazole is the main residue from the triazole label,
except in fat where flusilazole is the primary residue from the
triazole label.
The report
does not effectively answer questions raised by the 1989 and 1991
Meetings concerning differences in residues found between ruminant
and poultry metabolism and feeding studies. The Meeting noted
and agreed with the 1991 JMPR conclusion that these differences
probably result largely from the more detailed residue characterization
and identification in the poultry studies than in the ruminant
studies. The Meeting also agreed with the 1991 JMPR that although
all questions have not been completely answered, the nature of
the residue in animal products can be considered to be reasonably
well understood in view of the low residues expected (especially
for flusilazole) in animal products.
Soil dissipation.
The Meeting reviewed the final report of a 3-year soil dissipation
study (4 applications per year) for which an interim report was
reviewed by the 1989 JMPR. It confirms the 1989 observations that
over 92% of the radioactivity is confined to the top 8 cm of soil
over the test period, and that the predominant residues in this
segment are flusilazole and its silanol metabolite IN-F7321. The
author cites statistical evaluation of the data to support the
view that residues will reach a steady level at 57% of yearly
application levels after repeated application levels under worst-case
conditions.
The report
cites the steady-state conclusion, the strong adsorption to the
top layers of soil, the lack of residues exceeding 0.01 mg/kg
in the 24-36 cm soil depths and the weak leaching potential indicated
in other studies as evidence that residues in ground water were
unlikely. While the data indicate that over 92% of the radioactivity
remains in the top 8 cm of the silt loam soil investigated, and
indeed that residue levels are extremely low in the 24-36 cm depths,
it also shows an increasing penetration by low levels of radioactivity
over the test period in this soil type. The identity of these
residues in the deeper soil segments was not indicated.
While the
adsorption of this persistent pesticide to soil is strong, the
1989 JMPR had noted that uptake of low residue levels can occur
in rotational crops and that the leaching potential would be less
for silt loams (as in this study) than for more sandy soils. Because
the silt loam study was under worst-case conditions (bare ground,
repeated applications) and was consistent with reassuring findings
of a number of other relevant studies, the Meeting accepted that
ground water residues from silt loam soils were unlikely.
Freezer
storage stability. Instead of details of a previous 36.5-month
study for the parent compound only, the Meeting was provided with
a new 11-month freezer storage study of flusilazole and its metabolites
in wheat grain and straw. While the results suggest that about
30% of 0.3 mg/kg residues of the parent compound and its phenyl
metabolites in grain and straw are lost after various storage
intervals up to 11 months, the variability in the recoveries of
freshly fortified samples indicates that the apparent losses are
probably as much the result of analytical variability as actual
storage losses. The Meeting concluded that the data demonstrated
adequate stability of flusilazole and the metabolites IN-7321,
1,1,3,3-tetrakis(4-fluorophenyl)-1,3-dimethyldisiloxane (IN-G7072),
2-fluoro-5-[(4-fluorophenyl)(methyl)(1-H-1,2,4-triazol-1-ylmethyl)silyl]phenol
(IN-37722) and 2-fluoro-5-[(4-fluorophenyl)(hydroxy)(methyl)silyl]phenol
(IN-37738) (presumably unconjugated) over 11 months under the
conditions of the study.
The 11-month
storage interval compares with sampling-to-laboratory-receipt
intervals ranging from 2 to 15 months in cereal grain trials from
which data were reviewed by the 1989 JMPR. The Meeting did not
know the actual sampling-to-analysis intervals for the data reviewed
in 1989, although according to the 1989 monograph all samples
were generally stored at -20”C.
Cereals.
The original 1989 JMPR estimates of maximum residue levels of
0.1 and 2 mg/kg respectively for cereal grains and straws or fodders
(dry) were based on maximum residues of 0.07 mg/kg in grain and
1.7 mg/kg in straw. Although there were no outstanding requirements
for additional supervised trials data, the Meeting received extensive
additional cereal grain, plant, forage and straw data from Europe
and North America. Because no need for MRL revisions was indicated,
the Meeting only briefly summarized the submitted data on grain
and straw. It concluded that there was no need to revise the recently
adopted limits of 0.1 mg/kg in the grains and 2 mg/kg in the straws
and fodders (dry) of barley, rye and wheat at present. This conclusion
may need to be reconsidered at a future Meeting in the light of
future GAP information.
Cereal
grain processing. The 1991 JMPR reviewed a wheat processing
study submitted in response to a 1989 requirement. While no concentration
in milled fractions was observed, samples were not analysed for
metabolites (especially IN-F7321) and such analysis had been recorded
as desirable. A barley grain processing study provided to the
Meeting confirmed that no concentration of flusilazole or the
major metabolite IN-F7321 occurred in milling fractions.
Grapes.
Limited additional information on GAP in Europe and Australia
and additional grape data submitted in response to the 1991 requests
showed maximum residues reflecting GAP of 0.22 mg/kg compared
to the recently adopted CXL of 0.5 mg/kg. A delegation to the
CCPR had suggested that a 0.2 mg/kg limit was sufficient. The
Meeting confirmed the 1989 JMPR conclusion that residues were
unlikely to exceed 0.3 mg/kg.
Pome fruit.
Additional GAP information and residue data did not require a
revision of the current 0.2 mg/kg limit.
Stone
fruit. The 0.1 mg/kg limit for peaches and nectarines recommended
by the 1991 JMPR was temporary pending the submission of additional
GAP and residue data. It had been based on data from New Zealand
and France and GAP from New Zealand and Spain. The Meeting received
information on current GAP from Spain, France, Greece (pending)
and Italy, and residue data on nectarines from France and on peaches
from Australia, Italy, Greece, and the United States. French apricot
data were also provided as supporting information. No GAP information
was available for Australia or the United States. One to 4 applications
at 3-4 g ai/hl and a PHI of 7 to 10 days appears to be usual for
countries with established GAP, although in two cases the maximum
number of permitted applications was not indicated.
At a 7-day
PHI, the new French data or those summarized by the 1991 JMPR
which reflect GAP rates showed maximum residues of flusilazole
per se in peaches of 0.09 mg/kg (1991) or 0.08 mg/kg (1993), except
in one trial in the 1993 submission where a residue of 0.55 mg/kg
after 8 days was reported from 9 applications at GAP rates. Maximum
apricot residues reflecting GAP rates were 0.08 mg/kg after 7
days. Maximum residues in the US trials were 0.09 mg/kg at a 2.4
g ai/hl spray concentration after 7 or 14 days (0.2 mg/kg after
5 days) and 0.3 mg/kg at a 4.8 g ai/hl rate after 12 days. At
a pending GAP rate, maximum residues after 7 days in the Greek
trials were 0.09 mg/kg. Residues were not detected in the Australian
or Italian trials (<0.05 mg/kg and <0.01 mg/kg respectively),
but that is not unexpected in view of the long PHIs and the type
of application. The Meeting concluded that a 0.5 mg/kg limit was
supported for peaches. Observing that GAP for apricots and nectarines
is similar to that for peaches, the Meeting concluded that the
available data could also mutually support 0.5 mg/kg limits for
apricots and nectarines at a 7-day PHI.
Limited data
for plums and cherries were insufficient to recommend MRLs.
Sugar
cane. No residues (<0.02 mg/kg) were detected in the juice
from plants grown after dip treatments of sugar cane sets at fivefold
application rates. No stalks were analysed. The Meeting concluded
that the data were inadequate to support a limit for sugar cane.
FURTHER WORK
OR INFORMATION
Desirable
1. Submission
of analytical method AMR-115-85 cited in Du Pont, 1993, Vol. 1,
exhibit 6. Submission of validation information to permit estimation
of limits of determination is desirable.
2. On completion,
submission of the soil dissipation report AMR-791-87 (Fujinari,
1988). The interim report was reviewed by the 1989 JMPR.
|