|
Return to Epoxiconazole
Index Page
Activity: Fungicide
(conazole)
Structure:
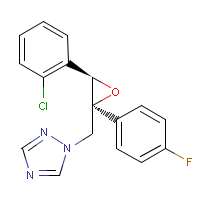
Adverse
Effects:
Body
Weight Decrease
Bone
Carcinogenic: LIVER
Endocrine: Adrenal
Endocrine: Ovary
Endocrine: Pituitary-Adrenal
axis
Endocrine: Suspected Disruptor
Liver
Environmental
"Epoxyconazole
106325-08-0*
Banned.
Low degradability, toxic to water-living organisms and endocrine
effects. 1997."
Definition: "Banned. A substance which for health or
environmental reasons by an authority decision is either
no longer approved for any area of application, or for which
an approval or registration has been denied from the first
instance."
Ref: Euopean Commission.
Appendix 5. Substances which may not be included as active
ingredients in approved pesticide products, Chapter 15,
Section 2, subsection one.
http://www.kemi.se/lagar_eng/pdf/app5_8.pdf
*
note from FAN: the CAS No. for Epoxiconazole has been changed
from 106325-08-0 to 135319-73-2. EPA also uses: 133855-98-8.
|
Body
Weight Decrease (click
on for all fluorinated pesticides)
iii. An oncogenicity
study in mice fed dosages of 0, 0.17, 0.81, 35.3, and 70.4 (males)
or 205.4 (females) mg/kg/day with a NOAEL of 0.81 mg/kg/day for
male and female mice based on the following effects: a. Highly
significant decreased body weights were observed in both
male and/or female mice at the mid-high and highest dose tested.
Ref: Federal Register: September 22, 2000.
[Page 57338-57344]. Notice of Filing Pesticide Petitions to Establish
Tolerances for Certain Pesticide Chemicals in or on Food.
http://www.fluoridealert.org/pesticides/Epoxiconazole.FR.Sept.2000.htm
Bone
(click on for all fluorinated
pesticides)
A developmental study
was conducted via oral gavage in rats resulted in dosages of 0,
5, 15, and 45 mg/kg/day HDT with a developmental toxicity NOAEL
of 5 mg/kg/day and a maternal toxicity of 5 mg/kg/day based on
the following:.. d. A significant
number of fetuses with skeletal variations
(especially rudimentary cervical
and/or accessory 14th rib(s)) in
the high dose group tested were observed. However, no malformations
were observed in any pups in this study. iii. In a second developmental
study in rats via dermal exposure for 6 hours/day on intact skin
with dosages of 0, 100, 400, and 1,000 mg/kg/day (HDT) with a
development toxicity NOAEL of 400 mg/kg/day and a maternal toxicity
of 400 mg/kg/day based on increased placental weights and a slight
increase in the number of fetuses with skeletal
variations was observed at the highest dose tested."
Ref: Federal Register. September 22, 2000.
[PF-961; FRL-6737-8].
http://www.fluoridealert.org/pesticides/Epoxiconazole.FR.Sept.2000.htm
Carcinogenic
(click
on for all fluorinated pesticides)
"Likely
to be Carcinogenic to Humans." Combined
hepatocellular tumors in male or female mice.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Endocrine:
Adrenal
(click
on for all fluorinated pesticides)
Endocrine disruption.
A series of mechanistic studies were performed to elucidate and
define the aromatase enzyme inhibition properties
of epoxiconazole. The following conclusions can be drawn from
the in vivo data: The effects on the ovaries are assessed to be
the result of the following: Decreasing aromatase enzyme activity
which is responsible for converting both testosterone and adrostendione
(male sex-steroids) into female sex steroids (e.g., estradiol).
This action would result in decreased estradiol (i.e., estrogen)
and increased androgen. As a consequence of reduced estradiol
levels, measured LH and FSH concentrations are slightly altered.
The increased incidences of neoplasms in the ovaries are considered
to be the result of a continuous cell proliferation by these stimulating
[[Page 57342]] hormones of the regulating hormones of the
pituitary-gonadal axis (LH and FSH). The changes adrenals
are assessed to be the result of the following: Decreasing
adrenal-cortical enzyme activity. This action would result
in decreased adrenal hormones such as corticosterone levels. As
a consequence of reduced corticosterone levels, pronounce ACTH
concentrations are found. The increased incidences of neoplasms
in the adrenals are considered to be the result of a continuos
cell proliferation by these stimulating hormones of the pituitary-adrenal
axis ACTH.
Ref: Federal Register: September 22, 2000
[Page 57338-57344]. Notice of Filing Pesticide Petitions to Establish
Tolerances for Certain Pesticide Chemicals in or on Food. http://www.fluoridealert.org/pesticides/Epoxiconazole.FR.Sept.2000.htm
Endocrine:
Ovary (click
on for all fluorinated pesticides)
Endocrine disruption.
A series of mechanistic studies were performed to elucidate and
define the aromatase enzyme inhibition properties of epoxiconazole.
The following conclusions can be drawn from the in vivo data:
The effects on the ovaries are assessed
to be the result of the following: Decreasing
aromatase enzyme activity which is responsible for converting
both testosterone and adrostendione (male sex-steroids) into female
sex steroids (e.g., estradiol). This action would result
in decreased estradiol (i.e., estrogen) and increased androgen.
As a consequence of reduced estradiol levels, measured LH and
FSH concentrations are slightly altered. The increased incidences
of neoplasms in the ovaries are considered
to be the result of a continuous cell proliferation by these stimulating
[[Page 57342]] hormones of the regulating hormones of the
pituitary-gonadal axis (LH and FSH). The changes adrenals are
assessed to be the result of the following: Decreasing adrenal-cortical
enzyme activity. This action would result in decreased adrenal
hormones such as corticosterone levels. As a consequence of reduced
corticosterone levels, pronounce ACTH concentrations are found.
The increased incidences of neoplasms in the adrenals are considered
to be the result of a continuos cell proliferation by these stimulating
hormones of the pituitary-adrenal axis ACTH.
Ref: Federal
Register: September 22, 2000 [Page 57338-57344]. Notice of Filing
Pesticide Petitions to Establish Tolerances for Certain Pesticide
Chemicals in or on Food. http://www.fluoridealert.org/pesticides/Epoxiconazole.FR.Sept.2000.htm
Endocrine:
Pituitary (click
on for all fluorinated pesticides)
Endocrine disruption.
A series of mechanistic studies were performed to elucidate and
define the aromatase enzyme inhibition properties of epoxiconazole.
The following conclusions can be drawn from
the in vivo data: The effects on the ovaries are assessed to be
the result of the following: Decreasing aromatase enzyme activity
which is responsible for converting both testosterone and adrostendione
(male sex-steroids) into female sex steroids (e.g., estradiol).
This action would result in decreased estradiol (i.e., estrogen)
and increased androgen. As a consequence of reduced estradiol
levels, measured LH and FSH concentrations are slightly altered.
The increased incidences of neoplasms in the ovaries are
considered to be the result of a continuous cell proliferation
by these stimulating [[Page 57342]] hormones of the regulating
hormones of the pituitary-gonadal axis
(LH and FSH). The changes adrenals are assessed to be the result
of the following: Decreasing adrenal-cortical
enzyme activity. This action would result in decreased adrenal
hormones such as corticosterone levels. As a consequence
of reduced corticosterone levels, pronounce ACTH concentrations
are found. The increased incidences of neoplasms in the adrenals
are considered to be the result of a continuos cell proliferation
by these stimulating hormones of the pituitary-adrenal
axis ACTH.
Ref: Federal Register: September 22, 2000
[Page 57338-57344]. Notice of Filing Pesticide Petitions to Establish
Tolerances for Certain Pesticide Chemicals in or on Food. http://www.fluoridealert.org/pesticides/Epoxiconazole.FR.Sept.2000.htm
Endocrine:
Suspected Disruptor (click
on for all fluorinated pesticides)
Suspected
Endocrine Disruptor
Ref: June 14, 2001 - Implementation of the
Community Strategy for Endocrine Disruptors - a range of substances
suspected of interfering with the hormone systems of humans and
wildlife. Communication from the Commission to the Council and
the European Parliament. Commission of the European Communities,
Brussels COM (2001) 262 final.
http://www.fluoridealert.org/pesticides/Endocrine.Disruptors.EC2001.pdf
(More information available at: http://europa.eu.int/eur-lex/en/com/cnc/2001/com2001_0262en01.pdf)
"Epoxyconazole
106325-08-0 Banned. Low degradability, toxic to water-living organisms
and endocrine effects. 1997."
Definition: "Banned. A substance which for health or environmental
reasons by an authority decision is either no longer approved
for any area of application, or for which an approval or registration
has been denied from the first instance."
Ref: Euopean Commission. Appendix 5. Substances
which may not be included as active ingredients in approved pesticide
products, Chapter 15, Section 2, subsection one.
http://www.kemi.se/lagar_eng/pdf/app5_8.pdf
Liver
(click on for all fluorinated
pesticides)
"Likely
to be Carcinogenic to Humans." Combined
hepatocellular tumors
in male or female mice.
Ref:
April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
-- Dose levels 23 mg/kg/day
resulted in maternal death, clinical signs, clinical chemical
effects, liver effects (i.e., damage),
histopathology, and limited number of pregnancy and pups with
reduced body weights which increased in severity to the upper
dose levels, this also indicated that doses above 23 mg/kg/day
were considered to be beyond the maximum tolerated dose (MTD)
for pregnant rats.
--Chronic toxicity. i. A series of two 1-year dog studies (study
A dose levels were 0, 1.6, 15, and 49 mg/kg/day for which a NOAEL
was established in females, and study B dose levels were 0, 0.3,
0.6, 0.9, and 1.1 mg/kg/day to determine a NOAEL in males. The
NOAEL was established as 1.1 mg/kg/day based on the following
effects: a. Mortality in the 49.0 mg/kg/day dose group with severe
clinical signs and evidence of liver
damage in those dogs which were sacrificed for humane reasons.
-- Separate chronic feeding and oncogenicity studies in rats were
performed to assess the chronic toxicity and oncogenic potential
of epoxiconazole. The chronic toxicity study was conducted at
dose levels of 0 and approximately 2, 8, 38, and 78 mg/ [[Page
57341]] kg/day. The oncogenicity study was conducted at dose levels
of 0 and approximately 2, 7, 40, and 80 mg/kg/day. The results
from the 2 studies are combined and summarized as follows: c.
Increased absolute and relative liver
weights were seen for males and/or females at dose levels 38 mg/kg/day.
d. Microscopic findings were observed in the
liver for male and/or female rats at dose levels 38 mg/kg/day,
in female adrenals at the highest dose test, and in the ovaries
at dose levels 38 mg/kg/day.
-- It has been determined that liver
tumor effects observed at the 70.4 and 205.4 mg/ kg/day dose levels
clearly exceeded the MTD. The liver
necrosis observed in the male and female mice, further support
the finding that the MTD was exceeded in the 70.4 and 205.4 mg/kg/day
dose levels. A series of mechanistic studies were performed to
elucidate and define the liver promotion
properties of epoxiconazole. The following conclusions can be
drawn from the data: The material is a potent
inducer of the hepatic cytocrome P-450 enzyme system, similar
to the drug-phenobarbital. The material induced proliferation
of the smooth endoplasmatic reticulum in the liver
centrolobular hypertrophy and induction of phase 1 and phase 2
enzymes of the xenobiotic metabolism. The
material was determined not to be an initiator of the carcinogenic
process, but a promoter of initiated
cells in the tumorgenesis as has been similarly shown with
drug--phenobarbital.
Ref:
September 22, 2000. Federal Register. [Notices] [Page 57338-57344].
Notice of Filing
Pesticide Petitions to Establish Tolerances for Certain Pesticide
Chemicals in or on Food.
http://www.fluorideaction.org/pesticides/epoxiconazole.fr.sept.2000.htm
|
Environmental
(click
on for all fluorinated pesticides)
"Epoxyconazole
106325-08-0*
Banned. Low degradability, toxic
to water-living organisms and endocrine effects.
1997."
Definition: "Banned.
A substance which for health or environmental reasons by
an authority decision is either no longer approved for any
area of application, or for which an approval or registration
has been denied from the first instance."
Ref: Euopean Commission.
Appendix 5. Substances which may not be included as active
ingredients in approved pesticide products, Chapter 15,
Section 2, subsection one.
http://www.kemi.se/lagar_eng/pdf/app5_8.pdf
*
note from FAN: the CAS No. for Epoxiconazole has been changed
from 106325-08-0 to 135319-73-2.
|
|
