|
Return
to Fluthiacet-methyl Index
Page
Activity: Herbicide
(unclassified)
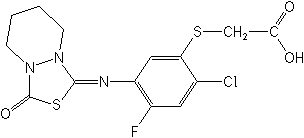
Structure for "Fluthiacet":
Adverse
Effects:
Anemia
Blood
Body
Weight Decrease
Bone
Cancer: Likely to be a human carcinogen -
PANCREAS (males) and LIVER
Clastogenic
Endocrine: Uterus
Liver
Pancreas
Environmental
As
of February, 2005, this herbicide is permitted in
or on 9 food commodities
in the United States - see list at bottom of page.
|
Anemia
(click on for all fluorinated pesticides)
Carcinogenicity in
rats. NOAEL males = 2.1 mg/kg/day LOAEL in males = 130 mg/kg/day
NOAEL in females = 2.5 mg/kg/day LOAEL in females = 154 mg/kg/day.
In males there were decreased body weight, liver toxicity,
pancreatic toxicity and microcytic anemia.
In females there were liver toxicity,
uterine toxicity and slight microcytic anemia.
In males only at 130 and 219 mg/kg/day there was respectively,
an increase in the trend toward pancreatic exocrine adenomas and
pancreatic islet cell adenomas.
Ref: Federal Register. December 21, 2001.
Fluthiacet-methyl; Pesticide Tolerance. Final Rule.
http://www.epa.gov/fedrgstr/EPA-PEST/2001/December/Day-21/p31497.htm
Blood
(click on for all fluorinated pesticides)
--
90-day oral Toxicity, rats and mice. Rats: NOAEL = 6.19 milligrams/
kilograms day (mg/ kg/day) in males 6.80 mg/ kg/day in females
LOAEL = 216 mg/ kg/day in males 249 mg/ kg/day in females Mice:
NOAEL = 1.3 mg/kg/ day in males 1.6 mg/ kg/day in females LOAEL
= 66 mg/kg/ day in males 83 mg/kg/ day in females. Based on decreased
body weight gains as well as effects
on hematology, clinical
chemistry, urinalysis
parameters, liver weights and microscopic pathology in rats; and
on effects on the erythropoietic system and liver in mice.
-- Carcinogenicity rats. NOAEL in males = 2.1 mg/kg/day LOAEL
in males = 130 mg/kg/day NOAEL in females = 2.5 mg/kg/day LOAEL
in females = 154 mg/kg/dayIn males there were
decreased body weight, liver toxicity, pancreatic toxicity
and microcytic anemia. In females
there were liver toxicity, uterine toxicity
and slight microcytic anemia.
In males only at 130 and 219 mg/kg/day there was respectively,
an increase in the trend toward pancreatic
exocrine adenomas and pancreatic islet cell adenomas.
-- In a 90-day rat feeding study the NOAEL was 6.19 mg/kg/day
for males and 6.80 mg/kg/day for females. In a 6-week dog dietary
study the NOAEL was 236 mg/kg/day for males and 77.7 mg/kg/day
for females. In a 28-day rat dermal study the NOAEL was 1000 mg/kg/day
[HDT]. In a 1-year dog chronic feeding study, the NOAEL was 57.6
mg/kg/day for males and 30.3 mg/kg/day for females and the Lowest
Observed Adverse Effect Level (LOAEL) was 582 mg/kg/day for males
and 145 mg/kg/day for females based on effects observed in the
erythropoietic system [red blood cells]
and the liver.
-- Chronic toxicity NOAEL in males = dogs 57.6 mg/kg/day LOAEL
in males = 582 mg/kg/day NOAEL in females = 30.3 mg/kg/day LOAEL
in females = 145 mg/kg/day The LOAELs were based on effects observed
in the erythropoietic system and
liver.
Ref: Federal Register: December
21, 2001. Fluthiacet-methyl; Pesticide Tolerance. Final Rule.
http://www.fluoridealert.org/pesticides/Fluthiacet.M.FR.Dec.21.2001.htm
Body
Weight Decrease (click
on for all fluorinated pesticides)
-- 90-day oral Toxicity,
rats and mice. Rats: NOAEL = 6.19 milligrams/ kilograms day (mg/
kg/day) in males 6.80 mg/ kg/day in females LOAEL = 216 mg/ kg/day
in males 249 mg/ kg/day in females Mice: NOAEL = 1.3 mg/kg/ day
in males 1.6 mg/ kg/day in females LOAEL = 66 mg/kg/ day in males
83 mg/kg/ day in females. Based on decreased
body weight gains as well as effects
on hematology, clinical
chemistry, urinalysis
parameters, liver weights and microscopic pathology in rats; and
on effects on the erythropoietic system and liver in mice.
-- 6-week oral toxicity in dogs. NOAEL = 236 mg/kg/ day in males
77.7 mg/ kg/day in females LOAEL = 709 mg/kg/ day in males 232
mg/ kg/day in females based on decreased
body weight gain.
-- 2-generation Reproduction and fertility effects. Parental/systemic
NOAEL = 1.59 mg/kg/ day in males LOAEL = 31.8 mg/kg/ day in males
NOAEL = 1.73 mg/kg/ day in females LOAEL = 35.2 mg/kg/ day in
females based on reduction in male body
weights/ gains and hepatic pathology Reproductive in males:
NOAEL = 31.8 mg/kg/ day LOAEL =313 mg/kg/ day Reproductive in
females: NOAEL = 37.1 mg/kg/ day LOAEL = 388 mg/kg/ day based
on decreases in mean litter body weights.
-- Carcinogenicity rats. NOAEL in males = 2.1 mg/kg/day LOAEL
in males = 130 mg/kg/day NOAEL in females = 2.5 mg/kg/day LOAEL
in females = 154 mg/kg/dayIn males there were decreased
body weight, liver toxicity, pancreatic toxicity and
microcytic anemia. In females there were liver toxicity,
uterine toxicity and slight microcytic anemia.
In males only at 130 and 219 mg/kg/day there was respectively,
an increase in the trend toward pancreatic exocrine adenomas and
pancreatic islet cell adenomas.
Ref: Federal Register: December 21, 2001.
Fluthiacet-methyl; Pesticide Tolerance. Final Rule. http://www.fluoridealert.org/pesticides/Fluthiacet.M.FR.Dec.21.2001.htm
Bone
(click
on for all fluorinated pesticides)
-- Prenatal
Maternal developmental in rats and rabbits. Maternal in rats:
NOAEL = 1,000 mg/ kg/day, HDT Maternal in rabbits: NOAEL = 1,000
mg/ kg/day, HDT Developmental in rabbits: NOAEL = 300 mg/kg/ day
Developmental in rabbits: LOAEL of 1,000 mg/ kg/day based on slight
non- significant increased incidence of irregularly
shaped sternebrae attributed to a delay in fetal development.
(See following.)
-- In rabbits, in utero exposure did not result in maternal toxicity
at 1,000 mg/kg/day. Developmental toxicity, however, was seen
at this dose characterized as an increase in irregular
sternebrae (a variation which is reversible). The occurrence
of developmental toxicity at which no maternal toxicity was noted
indicates an apparent increase in susceptibility. The Office of
Pesticide Program's Hazard Identification Assessment Review Committee
(HIARC), however, determined that the apparent susceptibility
is not convincing because of the equivocal nature of the effect
based on:
(1) The increased incidence of irregular sternebrae was not statistically
significant when compared to concurrent controls;
(2) the increase occurred at the Limit-Dose (1,000 mg/kg/day;
(3) it was the only anomaly seen (i.e., a single variation);
(4) the dose response was not strong because there was only a
small increase in the litter incidence between the low- (5 mg/kg/day)
and the high-dose (1,000 mg/kg/day), with the mid- and high-dose
groups having 8 litters with this variation;, and
(5) this endpoint is appropriate to establish a LOAEL and not
appropriate for risk assessments. Based on these factors, the
HIARC concluded that there is no increased susceptibility in the
rabbit study. Therefore, the 10X FQPA safety factor to ensure
the protection of infants and children was not applied in the
risk assessments.
Ref: Federal Register: December
21, 2001. Fluthiacet-methyl; Pesticide Tolerance. Final Rule. http://www.fluoridealert.org/pesticides/Fluthiacet.M.FR.Dec.21.2001.htm
Cancer:
Likely to be a human carcinogen - PANCREAS (males) and LIVER
(click on for all fluorinated pesticides)
Likely to
be Carcinogenic to Humans. Pancreatic
cell tumors (exocrine
adenomas, islet cell adenomas, and combined islet cell tumors);
Sprague-Dawley rats (M). Hepatocellular
tumors (adenomas and combined adenoma/carcinoma); CD-1
mice (M & F). CD-1 mice (M & F).
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Likely
to be carcinogenic to humans.
Reviewed 12/ 8/ 98.
Ref:
List of Chemicals Evaluated for Carcinogenic Potential. Science
Information Management Branch, Health Effects Division, Office
of Pesticide Programs, U. S. Environmental Protection Agency.
March 15, 2002.
http://www.biomuncie.org/chemicals_evaluated_for_carcinog.htm
-- Fluthiacet-methyl
is classified as a "likely to be a human
carcinogen" based on the presence of pancreatic
tumors (exocrine adenomas, islet cell adenomas and combined islet
cell adenomas + carcinomas) in male rats and liver
tumors (adenomas and combined adenomas + carcinomas) in male and
female mice.
Ref: US EPA. Pesticide Fact Sheet. Fluthiacet-methyl
Reason for Issuance: Conditional Registration Date Issued: April
1999.
http://www.epa.gov/opprd001/factsheets/fluthiacet.pdf
-- Carcinogenicity
rats. NOAEL in males = 2.1 mg/kg/day LOAEL in males = 130 mg/kg/day
NOAEL in females = 2.5 mg/kg/day LOAEL in females = 154 mg/kg/dayIn
males there were decreased body weight,
liver
toxicity, pancreatic
toxicity and microcytic anemia.
In females there were liver toxicity, uterine
toxicity and slight microcytic anemia. In males only at
130 and 219 mg/kg/day there was respectively, an increase in the
trend toward pancreatic exocrine adenomas
and pancreatic islet cell adenomas.
-- Carcinogenicity mice. NOAEL in males and females = 0.1 mg/
kg/day LOAEL in males and females = 0.1 and 1.2 mg/kg/day, respectively,
based on non- neoplastic liver findings.
In males, and possibly females, at 10 mg/kg/day for males and
12 mg/kg/day for females; and at 32 gm/kg/day for males and 37
mg/ kg/day for females, there was an increase in the number of
mice with hepatocellular adenomas, carcinomos
and or adenomas/ carcinomas.
-- Chronic Dietary General population. 18-month carcinogenicity
in the mouse. NOAEL = 0.1 mg/kg/day Non-neoplastic liver findings
(increase in absolute and relative liver
weights,
fatty changes, chronic inflammation, karyomegaly, single cell
necrosis and ceroid/lipofuscin pigmentation).
-- Cancer. Fluthiacet-methyl has been classified as ``likely
to be a human carcinogen'' by EPA. The Office of Pesticide
Programs, Heath Effects Division, Cancer Assessment Review Committee
recommended a linear low-dose approach (Q1*) for human risk assessment.
The Q1* is 0.207 (mg/kg/day)-1 in human equivalents and is based
upon the combined hepatocellular tumors (adenomas and carcinomas)
in male mice. EPA conducted a cancer assessment analysis
(food) using DEEM software and Tier 2 chronic dietary exposure
assumptions. The assumptions of this Tier 2 chronic dietary analysis
are as specified above. The cancer risk estimate (food only) for
the U.S. population (total) is 3.93 x 10-8. This risk estimate
translates to a dietary exposure of 1.90 x 10-7 mg/kg/day. This
dietary exposure value was back-calculated based upon the cancer
risk estimate and the Q1*. As cancer risk = Exposure x Q1 * Thus,
Exposure = cancer risk estimate/ Q1 * or Exposure = 3.93 x 10-8/0.207.
Ref: Federal Register: December 21, 2001.
Fluthiacet-methyl; Pesticide Tolerance. Final Rule. http://www.fluoridealert.org/pesticides/Fluthiacet.M.FR.Dec.21.2001.htm
Clastogenic
(click
on for all fluorinated pesticides)
-- Cytogenetics. In
vitro cytogenetic assays performed with two different mammalian
cell lines demonstrated that fluthiacet-methyl
is clastogenic both in the presence and absence of S9 activation.
Ref: Federal Register: December 21, 2001.
Fluthiacet-methyl; Pesticide Tolerance. Final Rule. http://www.fluoridealert.org/pesticides/Fluthiacet.M.FR.Dec.21.2001.htm
Endocrine:
Uterus
(click
on for all fluorinated pesticides)
-- Carcinogenicity
rats. NOAEL in males = 2.1 mg/kg/day LOAEL in males = 130 mg/kg/day
NOAEL in females = 2.5 mg/kg/day LOAEL in females = 154 mg/kg/dayIn
males there were decreased body weight,
liver toxicity, pancreatic toxicity and microcytic anemia. In
females there were liver toxicity, uterine
toxicity and slight microcytic anemia.
In males only at 130 and 219 mg/kg/day there was respectively,
an increase in the trend toward pancreatic
exocrine adenomas and pancreatic islet cell adenomas.
Ref: Federal Register: December 21, 2001.
Fluthiacet-methyl; Pesticide Tolerance. Final Rule. http://www.fluoridealert.org/pesticides/Fluthiacet.M.FR.Dec.21.2001.htm
Liver
(click
on for all fluorinated pesticides)
Likely to
be Carcinogenic to Humans. Pancreatic
cell tumors (exocrine
adenomas, islet cell adenomas, and combined islet cell tumors);
Sprague-Dawley rats (M). Hepatocellular
tumors (adenomas and combined adenoma/carcinoma); CD-1
mice (M & F). CD-1 mice (M & F).
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
A chronic reference
dose (RfD) (0.001 mg/kg/day) was identified for fluthiacet-methyl,
based on non-neoplastic liver findings
(increase in absolute and relative liver
weights, fatty changes, chronic inflammation, karyomegaly, single
cell necrosis and ceroid/lipofuscin pigmentation).
Ref. Federal Register. December 21, 2001.
Fluthiacet-methyl; Pesticide Tolerance. Final Rule.
http://www.epa.gov/fedrgstr/EPA-PEST/2001/December/Day-21/p31497.htm
-- Carcinogenicity rats.
NOAEL in males = 2.1 mg/kg/day LOAEL in males = 130 mg/kg/day
NOAEL in females = 2.5 mg/kg/day LOAEL in females = 154 mg/kg/dayIn
males there were decreased body weight,
liver
toxicity, pancreatic
toxicity and microcytic anemia. In
females there were liver toxicity, uterine
toxicity and slight microcytic anemia. In males only at
130 and 219 mg/kg/day there was respectively, an increase in the
trend toward pancreatic exocrine adenomas
and pancreatic islet cell adenomas.
-- Carcinogenicity mice. NOAEL in males and females = 0.1 mg/
kg/day LOAEL in males and females = 0.1 and 1.2 mg/kg/day, respectively,
based on non- neoplastic liver findings.
In males, and possibly females, at 10 mg/kg/day for males and
12 mg/kg/day for females; and at 32 gm/kg/day for males and 37
mg/ kg/day for females, there was an increase in the number of
mice with hepatocellular adenomas, carcinomos
and or adenomas/ carcinomas.
-- Chronic Dietary General population. 18-month carcinogenicity
in the mouse. NOAEL = 0.1 mg/kg/day Non-neoplastic liver findings
(increase in absolute and relative liver
weights,
fatty changes, chronic inflammation, karyomegaly, single cell
necrosis and ceroid/lipofuscin pigmentation).
-- Cancer. Fluthiacet-methyl has been classified as ``likely
to be a human carcinogen'' by EPA. The Office of Pesticide
Programs, Heath Effects Division, Cancer Assessment Review Committee
recommended a linear low-dose approach (Q1*) for human risk assessment.
The Q1* is 0.207 (mg/kg/day)-1 in human equivalents and is based
upon the combined hepatocellular tumors (adenomas and carcinomas)
in male mice. EPA conducted a cancer assessment analysis
(food) using DEEM software and Tier 2 chronic dietary exposure
assumptions. The assumptions of this Tier 2 chronic dietary analysis
are as specified above. The cancer risk estimate (food only) for
the U.S. population (total) is 3.93 x 10-8. This risk estimate
translates to a dietary exposure of 1.90 x 10-7 mg/kg/day. This
dietary exposure value was back-calculated based upon the cancer
risk estimate and the Q1*. As cancer risk = Exposure x Q1 * Thus,
Exposure = cancer risk estimate/ Q1 * or Exposure = 3.93 x 10-8/0.207.
Ref: Federal Register: December 21, 2001.
Fluthiacet-methyl; Pesticide Tolerance. Final Rule. http://www.fluoridealert.org/pesticides/Fluthiacet.M.FR.Dec.21.2001.htm
•
Definition:
Karyomegaly: the
condition of a cells nucleusbeing abnormally enlarged (i.e.,
for reasons other than it being polyploid).
Pancreas
(click
on for all fluorinated pesticides)
Likely to
be Carcinogenic to Humans. Pancreatic
cell tumors (exocrine
adenomas, islet cell adenomas, and combined islet cell tumors);
Sprague-Dawley rats (M). Hepatocellular
tumors (adenomas and combined adenoma/carcinoma); CD-1
mice (M & F). CD-1 mice (M & F).
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
-- Fluthiacet-methyl
is classified as a "likely to be a human
carcinogen" based on the presence of pancreatic
tumors (exocrine adenomas, islet cell adenomas and combined islet
cell adenomas + carcinomas) in male rats and liver
tumors (adenomas and combined adenomas + carcinomas) in male and
female mice.
Ref: US EPA. Pesticide Fact Sheet. Fluthiacet-methyl
Reason for Issuance: Conditional Registration Date Issued: April
1999.
http://www.epa.gov/opprd001/factsheets/fluthiacet.pdf
-- Carcinogenicity.
NOAEL in males = rats 2.1 mg/kg/day LOAEL in males = 130 mg/kg/day
NOAEL in females = 2.5 mg/kg/day LOAEL in females = 154 mg/kg/day.
In males there were decreased body weight, liver toxicity,
pancreatic toxicity and microcytic anemia. In females there
were liver toxicity, uterine toxicity and slight microcytic anemia.
In males only at 130 and 219 mg/kg/day there was respectively,
an increase in the trend toward pancreatic
exocrine adenomas and pancreatic islet cell adenomas.
Ref: Federal Register: December 21, 2001.
Fluthiacet-methyl; Pesticide Tolerance. Final Rule.
http://www.fluoridealert.org/pesticides/Fluthiacet.M.FR.Dec.21.2001.htm
| Environmental
(click
on for all fluorinated pesticides)
Phototoxic
Pesticide.
Light-dependent peroxidizing herbicides
(LDPHs). US EPA identified the following organofluorine
herbicides Acifluorfen, Azafenidin,
Carfentrazone-ethyl, Flumiclorac-penty, Flumioxazin, Fluthiacet-methyl,
Fomesafen, Lactofen, Oxadiargyl,
Oxadiazon, Oxyfluorfen, Sulfentrazone, Thidiazimin as
phototoxic pesticides that
act by inhibiting protoporphyringen oxidase in the heme
and chlorophyll biosynthetic pathway.
[10 out of the 13 pesticides that EPA identified are organofluorines].
SEE http://www.fluoridealert.org/pesticides/PHOTOTOXICITY.PAGE.htm
Ref: December 11, 2001 - US EPA. Revised
Environmental Fate and Effects Division Preliminary Risk
Assessment for the Oxyfluorfen Reregistration Eligibility
Decision Document - also at: http://www.epa.gov/oppsrrd1/reregistration/oxyfluorfen/oxyefedchap.pdf
--
Fluthiacet-methyl was shown to be practically non-toxic
to birds, practically non-toxic to small mammals, practically
non-toxic to bees and other beneficial insects, very
highly toxic to fish, and moderately toxic to fresh
water invertebrates. For seedling emergence, onion is
the most sensitive non-target plant species and for vegetative,
vigor, cucumber is the most sensitive non-target plant
species. For aquatic plants, duckweed and nonvascular
green algae are the most sensitive aquatic plant species.
There are no acute or chronic risk to non-target endangered
fish, birds, aquatic invertebrates, terrestrial or aquatic
plants or endangered species. Risks to endangered terrestrial
and aquatic species are expected to be minimal form the
use of Action Herbicide on soybeans. Because surfactant
is used with the end-use product, and may enhance the
product's phytotoxicity, limited vegetative vigor test
with onion, tomato, cucumber and an aquatic test with
duckweed are a condition of the registration for Action
Herbicide.
-- The following statement must appear in the Environmental
Hazards section of the label of end use products: This
pesticide is toxic to fish and aquatic invertebrates.
Do not discharge effluent containing this product into
lakes, streams, ponds, estuaries, oceans or other waters
unless in accordance with the requirements of a National
Pollutant Discharge Elimination System (NPDES) permit
and the permitting authority has been notified in writing
prior to discharge. Do not discharge effluent containing
this product to sewer systems without previously notifying
the sewage treatment plant authority. For guidance contact
your State Water Board or Regional Office of the Environmental
Protection Agency.
Ref: US EPA. Pesticide
Fact Sheet. Fluthiacet-methyl Reason for Issuance: Conditional
Registration Date Issued: April 1999. http://www.epa.gov/opprd001/factsheets/fluthiacet.pdf
|
A
February
16, 2005,
check at the Code
of Federal Regulations for Fluthiacet-methyl:
this herbicide is permitted in
or on 9 food
commodities in the United States.
The
following list identifies these crops for which EPA has set
pesticide.
|
| Code
of Federal Regulations]
[Title 40, Volume 22]
[Revised as of July 1, 2004]
From the U.S. Government Printing Office via GPO Access
[CITE: 40CFR180.551]
[Page 501]
TITLE 40--PROTECTION OF ENVIRONMENT
CHAPTER I--ENVIRONMENTAL PROTECTION AGENCY (CONTINUED)
PART 180_TOLERANCES AND EXEMPTIONS FROM TOLERANCES FOR PESTICIDE
CHEMICALS
IN FOOD--Table of Contents
Subpart C_Specific Tolerances
Sec. 180.551 Fluthiacet-methyl; tolerances for residues.
(a) General. A tolerance is established for residues of
the
herbicide, fluthiacet-methyl, acetic acid [[2-chloro-4-fluoro-5-
[(tetrahydro-3-oxo-1H,3H-[1,3,4]thiadiazolo[3,4-[alpha]]pyridazin-1-
ylidene)amino]phenyl]thio]-methyl ester, in or on the food
commodity: |
| Commodity |
Parts
per million |
| Corn,
field, forage |
0.050 |
| Corn,
field, grain |
0.010 |
| Corn,
field, stover |
0.050 |
| Corn,
pop, grain |
0.010 |
| Corn,
pop, stover |
0.050 |
| Corn,
sweet, forage |
0.050 |
| Corn,
sweet, kernel plus cob with husks removed |
0.010 |
| Corn,
sweet, stover |
0.050 |
| Soybean,
seed |
0.01 |
| (b)
Section 18 emergency exemptions. [Reserved]
(c) Tolerances with regional registrations. [Reserved]
(d) Indirect or inadvertent residues. [Reserved] |
|

