|
Return
to Index Page
Activity: Herbicide
(Aryloxyphenoxy
propionic acid)
Structure:
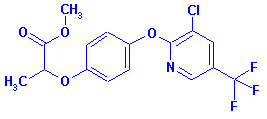
Adverse Effects:
Body Weight Decrease
Bone
Cancer:
Probable Human Carcinogen - LIVER
Cholesterol
Endocrine:
Testicular
Endocrine: Thyroid
Heart
Kidney
Liver
A
1992 Greenpeace
report contains case studies on 5 dangerous pesticides,
including this one, that have never been registered in the
US but are made in the US for export. Greenpeace argues for
an end to the loopholes which allow a double standard between
domestic and export pesticide.
|
Body
Weight Decrease (click
on for all fluorinated pesticides)
-- 1) 2-Year Feeding
(carcinogenicity) - mouse: Dietary levels tested:
0, 0.03, 0.065, and 0.6 mg/kg/day; B6C3F1 mice (50/sex/dose)
were administered haloxyfop-methyl in the diet for 24 months.
High-dose males exhibited a reduced body
weight gain, elevated alkaline phosphatase levels, and
an increase in relative liver weights. Histopathological
observations of the livers from high-dose males and females were
characterized by an alteration of the tinctorial staining properties
of the hepatocytes. Based on the above effects the LEL
for systemic toxicity is 0.6 mg/kg/day. The NOEL for systemic
toxicity is 0.065 mg/kg/day.; core grade minimum (Dow Chemical
U.S.A., 1985c)
-- 2-Generation Reproduction - rat: Dietary levels tested: 0,
0.01, 0.65, and 1.0 mg/kg/day; Groups Sprague-Dawley rats (30/sex/dose)
were administered haloxyfop-methyl. The F0 generation was dosed
for 8 weeks and the F1 generation for 11 weeks after each generation
was mated. The only apparent effect was reduced
body weights of the offspring in the F1a litters at weaning,
at 21 days in all treatment groups, and in the 1 mg/kg/day groups
of the F1b males and females and F2a males. Although
statistically significant, the reductions are not large, amount
to 10% or less. It is questionable whether this is a true
toxic effect in all exposure groups, since it was not seen in
subsequent litters except at the high-dose. No organ weights or
histopathological examinations were performed. No maternal or
reproductive toxicity was observed at any dose tested. The LEL
for developmental toxicity is 1 mg/kg/day based on reduced weanling
weight in F1a, F1b, and F2a litters. The NOEL for developmental
toxicity is 0.065 mg/kg/day.; core grade supplementary (Dow Chemical
U.S.A., 1985b)
-- 5) Developmental toxicity - rat: Dose levels tested: 0, 0.1,
1.0, 7.5, 10, and 25 mg/kg/day; Groups of pregnant Fischer 344
rats (10/dose) were administered haloxyfop-methyl orally during
days 6 through 15 of gestation. At the 7.5 mg/kg/day dose a decrease
in weight gain and food consumption accompanied by an increase
in water intake during gestation was observed. Additional maternal
toxicity was observed at 10 and 25 mg/kg/day,
including a decrease in weight gain and food consumption accompanied
by an increase in liver weight. An increase in the incidence of
resorptions was also observed at 10 and 25 mg/kg/day. At 7.5 mg/kg/day,
a significant incidence of delayed ossification of the centra
of the thoracic vertebra was observed.
The NOEL and LEL for maternal toxicity are 1 and 7.5 mg/kg/day,
respectively. The NOEL and LEL for developmental toxicity are
1 and 7.5 mg/kg/day, respectively; core grade guideline (Dow Chemical
U.S.A., 1983a)
-- 6) Developmental toxicity - rabbit: Dose levels tested: 0,
3, 7.5, and 15 mg/kg/day; Inseminated New Zealand White rabbits
(Dams: 27, 28, 30, and 25 for the control, low-, mid-, and high-dose,
respectively) were administered haloxyfop-methyl by gavage on
days 6 through 18 of gestation. No evidence of developmental toxicity
was observed at any dose tested. At the 7.5 mg/kg/day dose level
reduced body weight gain on days
6 to 18 was observed. Therefore the NOEL and LEL for maternal
toxicity is 3 and 7.5 mg/kg/day, respectively.; core grade minimum
(Dow Chemical Co., 1985)
-- 7) Developmental toxicity - rabbit: Dose levels tested: 0,
1.0, 7.5, and 20 mg/kg/day; Inseminated New Zealand White rabbits
(30/dose) were administered haloxyfop-methyl orally on days 6
through 18 of gestation. At 20 mg/kg/day, 4/31 pregnant animals
died between days 18 and 24 of gestation with 1/31 pregnant animals
dead on day 8 of gestation. A decrease in weight gain was also
observed at 20 mg/kg/day. Body weight gain at the 7.5 mg/kg/day
was comparable to controls. At 20 mg/kg/day, a significant increase
in the incidence of resorbed implantations was reported. The LEL
for maternal toxicity is 20 mg/kg/day based on dam mortality and
decreased weight gain. The NOEL for maternal toxicity is
7.5 mg/kg/day. The LEL for fetotoxicity is 20 mg/kg/day based
on the increase in resorptions. The NOEL for fetotoxicity is 7.5
mg/kg/day; core grade guideline (Dow Chemical U.S.A., 1983b)
Ref: Health Assessment. US EPA Integrated
Risk Information System (IRIS).
http://www.fluoridealert.org/pesticides/Haloxyfop.Methyl.IRIS.htm
Bone
(click
on for all fluorinated pesticides)
-- 5) Developmental
toxicity - rat: Dose levels tested: 0, 0.1, 1.0, 7.5, 10, and
25 mg/kg/day; Groups of pregnant Fischer 344 rats (10/dose) were
administered haloxyfop-methyl orally during days 6 through 15
of gestation. At the 7.5 mg/kg/day dose
a decrease in weight gain and food consumption accompanied by
an increase in water intake during gestation was observed. Additional
maternal toxicity was observed at 10 and 25 mg/kg/day, including
a decrease in weight gain and food consumption accompanied by
an increase in liver weight. An increase in the incidence of resorptions
was also observed at 10 and 25 mg/kg/day. At
7.5 mg/kg/day, a significant incidence of delayed ossification
of the centra of the thoracic vertebra was observed. The
NOEL and LEL for maternal toxicity are 1 and 7.5 mg/kg/day, respectively.
The NOEL and LEL for developmental toxicity are 1 and 7.5 mg/kg/day,
respectively; core grade guideline (Dow Chemical U.S.A., 1983a)
Ref:
Health Assessment. US EPA Integrated Risk Information System (IRIS).
http://www.fluoridealert.org/pesticides/Haloxyfop.Methyl.IRIS.htm
Cancer:
Possible Human Carcinogen - LIVER
(click
on for all fluorinated pesticides)
Group B --
Probable Human Carcinogen. Liver tumors
[adenomas (M), carcinomas (F) & adenomas/carcinomas
(M & F)]; B6C3F1 mice.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Group B2--Probable Human Carcinogen.
Reviewed 9/ 18/ 89.
Ref: List of Chemicals Evaluated for Carcinogenic
Potential. Science Information Management Branch, Health Effects
Division, Office of Pesticide Programs, U. S. Environmental Protection
Agency. March 15, 2002.
http://www.biomuncie.org/chemicals_evaluated_for_carcinog.htm
There are eight diphenyl
ethers that are structurally similar to diclofop-methyl. Of the
chemicals, fomesafen sodium, haloxyfop-methyl
(Verdict), oxyfluorfen, acifluorfen
sodium, nitrofen, and lactofen were reviewed in the initial
CPRC report. All of these chemicals induced
liver adenomas and carcinomas in rats and/or mice. Except
for haloxyfop-methyl, all of the other chemicals
produced positive results in at least one of the mutagenicity
assays...
May
24, 2000 - Cancer
Assessment Document. Evaluation of the
Carcinogenic Potential of Diclofop-Methyl. (Second Review). Final
Report. Cancer Assessment Review Committee, Health Effects Division,
US EPA Office of Pesticide Programs.
Note:
Except for Nitrofen, all the pesticides cited above are fluorinated.
Cholesterol
(click
on for all fluorinated pesticides)
-- 13-Week Feeding
- dog: Dietary levels tested: 0, 2, 5, and 20 mg/kg/day; Beagle
dogs (4/sex/dose level) were administered haloxyfop-methyl in
the diet for 13 weeks. A statistically significant
decrease in serum cholesterol values was reported for males
fed 2 mg/kg/day. A statistically significant
decrease in serum cholesterol values was reported for males
and females fed 5 mg/kg/day. A significant
decrease in male and female triiodothyronine and free thyroxine
values was accompanied by a significant decrease in male and female
relative thyroid/parathyroid weights. Hepatic peroxisomal fatty
acid beta-oxidation was increased in males and females fed 5 mg/kg/day.
Histological changes reported at this level were hepatocellular
enlargement with increased glycogen content, decrease in follicular
size and hypertrophy of the follicular epithelial cells of the
thyroid, and decrease size of the testicular tubules. The LEL
for systemic toxicity is 2 mg/kg/day, the lowest dose tested,
based on decreases in serum cholesterol values in males. A NOEL
for systemic toxicity was not established; core grade minimum
(Dow Chemical Co., 1987a)
Ref: Health Assessment. US EPA Integrated
Risk Information System (IRIS).
http://www.fluoridealert.org/pesticides/Haloxyfop.Methyl.IRIS.htm
Endocrine:
Testicular
(click on for all fluorinated
pesticides)
-- 2) 13-Week Feeding
- dog: Dietary levels tested: 0, 2, 5, and 20 mg/kg/day; Beagle
dogs (4/sex/dose level) were administered
haloxyfop-methyl in the diet for 13 weeks. A statistically significant
decrease in serum cholesterol values was reported for males fed
2 mg/kg/day. A statistically significant decrease in serum cholesterol
values was reported for males and females fed 5 mg/kg/day. A significant
decrease in male and female triiodothyronine and free thyroxine
values was accompanied by a significant decrease in male and female
relative thyroid/parathyroid weights. Hepatic peroxisomal fatty
acid beta-oxidation was increased in males and females fed 5 mg/kg/day.
Histological changes reported at this level were hepatocellular
enlargement with increased glycogen content, decrease in follicular
size and hypertrophy of the follicular epithelial cells of the
thyroid, and decrease size of the
testicular tubules. The LEL for systemic toxicity is 2
mg/kg/day, the lowest dose tested, based on decreases in serum
cholesterol values in males. A NOEL for systemic toxicity was
not established; core grade minimum (Dow Chemical Co., 1987a)
-- 4) 16-Week Feeding - rat: Dietary levels tested: 0, 0.002,
0.02, 0.2, and 2 mg/kg/day; CDF Fischer 344 rats (15/sex/group)
were administered haloxyfop- methyl in the
diet for 16 weeks. A significant dose-related increase in relative
liver weight was reported for male rats of the 0.002, 0.02, 0.2,
and 2.0 mg/kg/day levels by 4, 5, 11, and 44%, respectively. Female
relative liver weights were increased significantly (4%) at the
2.0 mg/kg/day levels. Males fed the 0.2 and 2.0 mg/kg/day levels
showed enlarged hepatocytes and increased cytoplasmic homogeneity.
An increase in hepatocellular cytoplasmic homogeneity was reported
for females at 2.0 mg/kg/day. A significant
decrease in relative testes weight (5%) accompanied by atrophy
of the seminiferous tubules were reported for males fed the 2.0
mg/kg/day level. The LEL for systemic
toxicity is 0.002 mg/kg/day, the lowest dose tested, based on
a dose related increase in relative liver weight in males. A NOEL
for systemic toxicity was not established.; core grade minimum
(Dow Chemical U.S.A., 1982a)
-- Critical Effect: Reduced relative kidney
weights in F0, F1, and F2b adults; Reduced
fertility in the F1/F2b generation. 3-Generation Rat Reproduction
Study. Dow Chemical U.S.A.,1985a. NOEL: 0.005 mg/kg/day; LEL:
0.05 mg/kg/day....
Ref:
Health Assessment. US EPA Integrated Risk Information System (IRIS).
http://www.fluoridealert.org/pesticides/Haloxyfop.Methyl.IRIS.htm
Endocrine:
Thyroid
(click on for all fluorinated
pesticides)
-- 2) 13-Week Feeding
- dog: Dietary levels tested: 0, 2, 5, and 20 mg/kg/day; Beagle
dogs (4/sex/dose level) were administered
haloxyfop-methyl in the diet for 13 weeks. A statistically significant
decrease in serum cholesterol values was reported for males fed
2 mg/kg/day. A statistically significant decrease in serum cholesterol
values was reported for males and females fed 5 mg/kg/day.
A significant decrease in male and
female triiodothyronine and free thyroxine
values was accompanied by a significant decrease in male and female
relative thyroid/parathyroid weights. Hepatic peroxisomal
fatty acid beta-oxidation was increased in males and females fed
5 mg/kg/day. Histological changes reported at this level were
hepatocellular enlargement with increased
glycogen content, decrease in follicular size and hypertrophy
of the follicular epithelial cells of the thyroid, and decrease
size of the testicular tubules. The LEL for systemic toxicity
is 2 mg/kg/day, the lowest dose tested, based on decreases in
serum cholesterol values in males. A NOEL for systemic toxicity
was not established; core grade minimum (Dow Chemical Co., 1987a)
Ref:
Health Assessment. US EPA Integrated Risk Information System (IRIS).
http://www.fluoridealert.org/pesticides/Haloxyfop.Methyl.IRIS.htm
Heart
(click on for
all fluorinated pesticides)
-- 7) 36-Week Feeding
- mouse: Dietary levels tested: 0, 0.02, and 2.0 mg/kg/day; B6C3F1
mice (10/sex/group) were administered haloxyfop-methyl in the
diet for 9 months. A significant increase in serum alkaline phosphatase
was reported for males at the 2.0 mg/kg/day level with a slight
increase in serum alkaline phosphatase for females. The liver
was slightly enlarged and darkened for both males and females
at 2.0 mg/kg/day. A significant increase
in the liver absolute weight and organ-to-body weight ratio of
both males and females fed 2.0 mg/kg/day was observed.
Males also exhibited a significant decrease in kidney and heart
weights compared with the control organ
weight. Livers of males and
females at the 2.0 mg/kg/day dose exhibited an enlargement of
centrilobular hepatocytes cells with an increase cytoplasmic homogenity
and increased eosinophilia. Kidneys of males fed 2.0 mg/kg/day
showed a decrease of cytoplasmic vacuolation of the proximal convoluted
tubular cells. Based on the above effects, the LEL for
systemic toxicity is 2.0 mg/kg/day. The NOEL for systemic toxicity
is 0.02 mg/kg/day; core supplementary (Dow Chemical U.S.A., 1982d)
Ref:
Health Assessment. US EPA Integrated Risk Information System (IRIS).
http://www.fluoridealert.org/pesticides/Haloxyfop.Methyl.IRIS.htm
Kidney
(click on for all fluorinated
pesticides)
-- 3) 13-Week Feeding
- monkey: Dietary levels tested: 0, 2, 10, and 30 mg/kg/day; Cynomolgus
monkeys (4/sex/dose level) were administered haloxyfop- methyl
by nasogastric intubation for 13 weeks. A statistically significant
decrease in triglyceride values was reported for males and females
dosed at 2 mg/kg/day. The statistically significant decrease in
triglyceride values in males and females dosed at 10 mg/kg/day
was accompanied by a nonsignificant decrease (15%) in cholesterol
values for females at this level. Female livers appeared pale
with an accentuated lobular pattern. Slight hepatocellular hypertrophy
was observed for male and females at this level. Relative
kidney weights were significantly increase for males and females
by 12 and 37%, respectively. The LEL for systemic toxicity
is 2 mg/kg/day, the lowest dose tested, based on a statistically
significant decrease in triglyceride values in males and females.
A NOEL for systemic toxicity was not established; core grade minimum
(Dow Chemical Co., 1987b)
-- 7) 36-Week Feeding - mouse: Dietary levels tested: 0, 0.02,
and 2.0 mg/kg/day; B6C3F1 mice (10/sex/group) were administered
haloxyfop-methyl in the diet for 9 months. A significant increase
in serum alkaline phosphatase was reported for males at the 2.0
mg/kg/day level with a slight increase in serum alkaline phosphatase
for females. The liver was slightly enlarged and darkened for
both males and females at 2.0 mg/kg/day.
A significant increase in the liver absolute weight and
organ-to-body weight ratio of both males and females fed 2.0 mg/kg/day
was observed. Males also exhibited a significant
decrease in kidney and heart weights compared with the control
organ weight. Livers of males and
females at the 2.0 mg/kg/day dose exhibited an enlargement of
centrilobular hepatocytes cells with an increase cytoplasmic homogenity
and increased eosinophilia. Kidneys
of males fed 2.0 mg/kg/day showed a decrease of cytoplasmic vacuolation
of the proximal convoluted tubular cells. Based on the
above effects, the LEL for systemic toxicity is 2.0 mg/kg/day.
The NOEL for systemic toxicity is 0.02 mg/kg/day; core supplementary
(Dow Chemical U.S.A., 1982d)
-- Critical Effect: Reduced relative kidney
weights in F0, F1, and F2b adults; Reduced
fertility in the F1/F2b generation. 3-Generation Rat Reproduction
Study. Dow Chemical U.S.A.,1985a. NOEL: 0.005 mg/kg/day; LEL:
0.05 mg/kg/day.... Signs of toxicity in parental rats at 1 mg/kg/day
level were reduced body weight gain and reduced food consumption
without increased mortality or obvious toxicity to the offspring.
In addition, a significant increase in relative liver weight and
enlarged livers were observed, however this finding was more frequent
in males than females. A significant
decrease in relative kidney weight was observed at 0.05
and 1 mg/kg/day, but it again occurred more
frequently in the F0, F1, and F2b adult
male rats. Renal pigmentation was also reported at 1 mg/kg/day
for male and female adult rats after the gross and histopathological
examinations. Based on decreases in relative kidney weights, the
LEL for systemic toxicity is 0.05 mg/kg/day. The NOEL for systemic
toxicity is 0.005 mg/kg/day.
-- 4) 2-Year Feeding (carcinogenicity) - rat: Dietary levels tested:
Male: 0, 0.01, 0.03, 0.065, and 0.1 mg/kg/day; Female: 0, 0.01,
0.03, 0.065, and 1.0 mg/kg/day; CDF Fischer 344 rats (50/sex/dose)
were administered haloxyfop- methyl in the diet for 2 years. No
effects were observed in the male at any dose tested. The LEL
for systemic toxicity is 1 mg/kg/day based on a significant
decrease in female absolute (8%) and relative (9%) kidney weights
accompanied by a significant increase in the incidence (24/50)
in renal pigmentation reported for females of this level.
Kidney function was not impaired as the urinalysis parameters
between test and control values were comparable. The NOEL for
systemic toxicity is 0.065 mg/kg/day; core grade guideline for
chronic toxicity (Dow Chemical U.S.A., 1984b)
Ref:
Health Assessment. US EPA Integrated Risk Information System (IRIS).
http://www.fluoridealert.org/pesticides/Haloxyfop.Methyl.IRIS.htm
Liver
(click
on for all fluorinated pesticides)
Group
B -- Probable Human Carcinogen.
Liver tumors [adenomas (M), carcinomas
(F) & adenomas/carcinomas (M & F)]; B6C3F1 mice.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Group B2--Probable Human Carcinogen.
Reviewed 9/ 18/ 89.
Ref: List of Chemicals Evaluated for Carcinogenic
Potential. Science Information Management Branch, Health Effects
Division, Office of Pesticide Programs, U. S. Environmental Protection
Agency. March 15, 2002.
http://www.biomuncie.org/chemicals_evaluated_for_carcinog.htm
"There are eight
diphenyl ethers that are structurally similar to diclofop-methyl.
Of the chemicals, fomesafen sodium, haloxyfop-methyl
(Verdict), oxyfluorfen, acifluorfen
sodium, nitrofen, and lactofen were reviewed in the initial
CPRC report. All of these chemicals induced
liver adenomas and carcinomas in rats and/or mice..."
Ref: May 24, 2000 - Cancer Assessment Document.
Evaluation of the Carcinogenic Potential of Diclofop-Methyl. (Second
Review). Final Report. Cancer Assessment Review Committee, Health
Effects Division, US EPA Office of Pesticide Programs.
http://www.fluoridealert.org/pesticides/cancer.epa.assess.may.2000.pdf
-- 1) 2-Year Feeding
(carcinogenicity) - mouse: Dietary levels tested: 0, 0.03, 0.065,
and 0.6 mg/kg/day; B6C3F1 mice (50/sex/dose) were administered
haloxyfop-methyl in the diet for 24 months. High-dose males exhibited
a reduced body weight gain, elevated
alkaline phosphatase levels, and an increase
in relative liver weights. Histopathological observations
of the livers from high-dose males and females were characterized
by an alteration of the tinctorial staining
properties of the hepatocytes. Based on the above effects
the LEL for systemic toxicity is 0.6 mg/kg/day. The NOEL for systemic
toxicity is 0.065 mg/kg/day.; core grade minimum (Dow Chemical
U.S.A., 1985c)
-- 4) 16-Week Feeding - rat: Dietary levels tested: 0, 0.002,
0.02, 0.2, and 2 mg/kg/day; CDF Fischer 344 rats (15/sex/group)
were administered haloxyfop- methyl in the diet for 16 weeks.
A significant dose-related increase in relative liver weight was
reported for male rats of the 0.002, 0.02, 0.2, and 2.0
mg/kg/day levels by 4, 5, 11, and 44%, respectively. Female relative
liver weights were increased significantly (4%) at the 2.0 mg/kg/day
levels. Males fed the 0.2 and 2.0 mg/kg/day levels showed enlarged
hepatocytes and increased cytoplasmic homogeneity. An increase
in hepatocellular cytoplasmic homogeneity was reported for females
at 2.0 mg/kg/day. A significant decrease
in relative testes weight (5%) accompanied by atrophy of the seminiferous
tubules were reported for males fed the 2.0 mg/kg/day level. The
LEL for systemic toxicity is 0.002 mg/kg/day, the lowest dose
tested, based on a dose related increase
in relative liver weight in males. A NOEL for systemic
toxicity was not established.; core grade minimum (Dow Chemical
U.S.A., 1982a)
-- 7) 36-Week Feeding - mouse: Dietary levels tested: 0, 0.02,
and 2.0 mg/kg/day; B6C3F1 mice (10/sex/group) were administered
haloxyfop-methyl in the diet for 9 months. A significant increase
in serum alkaline phosphatase was reported for males at the 2.0
mg/kg/day level with a slight increase in serum alkaline phosphatase
for females. The liver was slightly enlarged and darkened for
both males and females at 2.0 mg/kg/day. A
significant increase in the liver absolute weight and organ-to-body
weight ratio of both males and females fed 2.0 mg/kg/day was observed.
Males also exhibited a significant decrease in kidney and heart
weights compared with the control organ weight. Livers
of males and females at the 2.0 mg/kg/day dose exhibited an enlargement
of centrilobular hepatocytes cells with an increase cytoplasmic
homogenity and increased eosinophilia. Kidneys
of males fed 2.0 mg/kg/day showed a decrease of cytoplasmic vacuolation
of the proximal convoluted tubular cells.
Based on the above effects, the LEL for systemic toxicity
is 2.0 mg/kg/day. The NOEL for systemic toxicity is 0.02 mg/kg/day;
core supplementary (Dow Chemical U.S.A., 1982d)
-- Critical Effect: Reduced relative kidney
weights in F0, F1, and F2b adults; Reduced fertility in
the F1/F2b generation. 3-Generation Rat Reproduction Study. Dow
Chemical U.S.A.,1985a. NOEL: 0.005 mg/kg/day; LEL: 0.05 mg/kg/day....
Signs of toxicity in parental rats at 1 mg/kg/day level
were reduced body weight gain and reduced food consumption without
increased mortality or obvious toxicity to the offspring. In addition,
a significant increase in relative
liver weight and enlarged livers were observed, however
this finding was more frequent in males
than females. A significant decrease
in relative kidney weight was observed at 0.05 and 1 mg/kg/day,
but it again occurred more frequently in the F0, F1, and F2b adult
male rats. Renal pigmentation was also reported at 1 mg/kg/day
for male and female adult rats after the gross and histopathological
examinations. Based on decreases in relative kidney weights, the
LEL for systemic toxicity is 0.05 mg/kg/day. The NOEL for systemic
toxicity is 0.005 mg/kg/day.
Ref:
Health Assessment. US EPA Integrated Risk Information System (IRIS).
http://www.fluoridealert.org/pesticides/Haloxyfop.Methyl.IRIS.htm
|

