|
Return to Fluvalinate
Adverse Effects
ACTIVITY:
Acaricide; Insecticide
(pyrethroid)
See also:
Tau-fluvalinate
CAS NAME:
cyano(3-phenoxyphenyl)methyl N-[2-chloro-4-(trifluoromethyl)phenyl]-DL-valinate
NOTES:
One subset of isomers of this substance has its own ISO common name;
see tau-fluvalinate.
Structure:
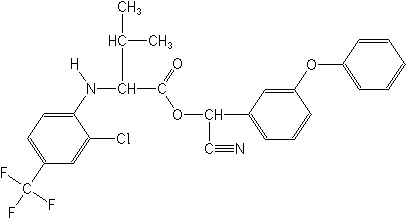
| Adverse
Effects:
Anemia
Blood
Body
Weight Decrease
Bone
Brain
Dermal
Endocrine:
Spermatogenesis
Endocrine
Disruptor: Suspected |
| Environmental
Effects:
Very
highly toxic to Crustaceans and Fish
Highly
toxic to Zooplankton |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Yes |
| US
EPA PC Code: |
109302 |
| US
Tolerances: |
CFR
180.427
CFR 186.3400 |
| FDA
LMS Code: |
297 |
US
EPA Permit Date
and Registrant: |
1983,
Zoecon Corp. |
Registered
use in
(includes only a limited list of countries)
|
Canada,
Japan, NZ, US |
| Japan's
Maximum Residue Levels (MRLs) |
Partial
list: Apple, Apricot, Barley,
Buckwheat, Cabbage, Carrot, Cauliflower, Cherry, Chestnut,
Cucumber, Egg Plant, Garlic, Grape, Grapefruit, Hop, Kiwifruit,
Lemon, Lettuce, Lime, Nectarine, Onion, Orange, Peach, Pear,
Peas, Potato, Rice, Rye, Strawberry, Tea, Tomato, Yam
--Note
high levels - 10 ppm - for Tea
(Green, Black, Oolong, Wulung) |
US
Maximum Residue Levels permitted
in food commodities
|
Honey
--
Note that several food tolerances were cancelled between 2002
and 2003 |
| Other
Information |
| Molecular
Formula: |
C26H22Cl
F3 N2O3 |
| Manufacturers: |
BAYER
Corp.
Sandoz Agro |
| Other
Names: |
Klartan
Mavrik
Mavrik Aqua Flow
Spur
Yardex
NISSO MAVRIC JET |
| Manufacture
site: |
US:
Bayer
/ Sandoz Inc, Beaumont, Texas 77705 |
| Of
special interest: |
| PAN
BAD ACTOR - Developmental
Toxin |
2005
- Schedule for Reregistration & Tolerance Reassessment (RED)
is expected to be May 2006. Contact at EPA: Kylie Rothwell (703)
308-8055; rothwell.kylie@epa.gov . According to EPA:
Through the pesticide reregistration and tolerance reassessment
programs, EPA is assessing risks and making risk management
decisions for older pesticides. These decisions are summarized
in documents known as REDs, IREDs, and TREDs. By making decisions
according to the schedule below, EPA will meet its statutory
deadlines for completing reregistration and tolerance reassessment.
Some of the decision dates presented in the schedule may change
due to the dynamic nature of the review process. Any pesticide
decisions that are not completed during the current fiscal year
will be rescheduled for the following year. EPA is committed
to meeting its reregistration and tolerance reassessment deadlines.
http://www.epa.gov/pesticides/reregistration/decision_schedule.htm
|
| TOXNET
profile from Hazardous Substances Data Bank |
| Identified
in the State of California as "Known to Cause Developmental
Toxicity" - Prop 65 |
| 2002
- NIPON
SODA ANNUAL REPORT 2002
- cites the following organofluorine pesticides
and their product names: Chlorfenapyr * Fluacrypyrim * Fluazinam
* Fluvalinate * Triflumizole |
| September
2001.
Draft
Toxicological Profile for Pyrethrins and Pyrethroids. Organofluorine
pyrethroids discussed in the Profile: Bifenthrin (Type 1), Cyfluthrin
(Type 2), Cyhalothrin (Type 2), Flucythrinate (Type 2), Flumethrin
(Type 2), Fluvalinate (Type 2),
Tefluthrin (Type 1). US Department of Health and Human Services.
Public Health Service Agency for Toxic Substances and Disease
Registry. |
| June
14, 2001 - Implementation
of the Community Strategy for Endocrine Disruptors Communication
from the Commission to the Council and the European Parliament.
Commission of the European Communities, Brussels COM (2001)
262 final. |
| Pesticide
Information Profile - Cornell University
Extoxnet. |
| April
2000 -
Food and Drug Administration
Pesticide Residue Monitoring. - Table
3. Pesticides detectable by methods used in 1999 regulatory
monitoring. |
| October
1998 - Structural
Pest Management pesticides. FAN's
compilation of information cited on fluorine and organofluorine
pesticides published in General Pest Management, Category 7A.
A Guide for Commercial Applicators. Prepared by: Carolyn Randall,
MSU Pesticide Education Program. Published by
MSU Pesticide Education
(Michigan State University). MSU
manual number: E-2048. |
| March
30, 1994 -
Temporary Registration
of Fluvalinate-Tau for Honey Bee Mite Detection and/or Control.
Decision Document. - Environment Canada |
| Believed
to be obsolete or discontinued for use as pesticides (US allows
residues in honey) .
WHO
Recommended Classification of Pesticides by Hazard and Guidelines
to Classification 2000-2002. Table
6. Active ingredients not included in the Classification and
believed to be obsolete or discontinued for use as pesticides,
p 37. Note from EC: According to Pesticide Standard Limits in
Japan (see above), Fluvalinate is allowed for use. Also, USEPA
lists fluvalinate as an active ingredient. See "Active
Products" at http://www.cdpr.ca.gov/cgi-bin/epa/chemdetiris.pl?pccode=109302
Due to these discrepancies asked for a
clarification (on Dec 17, 2001) from WHO.
The reply was that they assume it is no longer being used. |
Rationale
for US EPA to add Fluvalinate to the Toxic Release Inventory
Delayed
ossification and decreased weight and length of fetuses
were observed in offspring of rats orally administered 50
mg/kg/day (LOEL) on days 6 to 15 of gestation. The NOEL
was 10 mg/kg/day. These effects were observed at doses that
produced maternal toxicity. Curved tibia and fibula were
observed in the offspring of rabbits orally administered
125 mg/kg/day (LOEL). The NOEL was 25 mg/kg/day. In a 2-generation
reproduction study, a decrease in pup weight and growth
were observed in offspring of rats orally administered 5
mg/kg/day (LOEL). The NOEL was 1 mg/kg/day. Significantly
decreased weight and survival were observed in offspring
of rats orally administered 25 mg/kg/day.
In a range finding study, dietary administration of 50 mg/kg/day
for 30 days produced skin lesions in rats. The NOEL was
not determined. A 2-year rat feeding study was terminated
at 64 weeks due to dermal lesions produced in animals at
15 mg/kg/day. The NOEL was 2 mg/kg/day. Dietary administration
of 10 mg/kg/day (LOEL for effect) to mice for 2 years produced
scabbing and dermal abrasion. No NOEL for these effects
was established. An increase in plantar ulcers was observed
in rats fed 2.5 mg/kg/day (LOEL) for 2 years. The NOEL was
1 mg/kg/day. Decreases in body weight gain were also observed
in this study. Based on the NOEL of the study, an oral RfD
of 0.01 mg/kg/day was derived. In a 2- generation rat reproduction
study, dietary administration of 5 mg/kg/ day produced decreased
body weight gain and skin lesions in parents and offspring.
Dietary administration of 2.5 mg/kg/day to rats for 13 weeks
produced anemia in blood parameters (decreased hematocrit,
hemaglobin, and red blood cells). The NOEL was 1.0 mg/kg/day.
Dietary
administration of 30 mg/kg/day (LOEL) to rats for 3 months
produced decreased hemoglobin, hematocrit, and red blood
cell count in rats. The NOEL was 3 mg/kg/day.
EPA believes that there is sufficient evidence for listing
fluvinate on EPCRA section 313 pursuant to EPCRA section
313(d)(2)(B) based on the available developmental, dermal,
and hematological toxicity data for this chemical.
Aquatic
acute toxicity values for fluvalinate include a daphnid
48- hour EC 50 of 0.40 ppb, a bluegill sunfish 96-hour LC50
of 0.9 ppb, a rainbow trout 96-hour LC50 of 2.9 ppb,
and a sheepshead minnow 96-hour LC 50 of 10.8 ppb.
EPA believes that there is sufficient evidence for listing
fluvinate on EPCRA section 313 pursuant to EPCRA section
313(d)(2)(C) based on the available environmental toxicity
data for this chemical.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals
from Federal Insecticide, Fungicide, Rodenticide Act (FIFRA)
Active Ingredients to EPCRA Section 313. U. S. Environmental
Protection Agency, Washington, DC (1993).
As cited by US EPA in:
Federal
Register: January 12, 1994. Part
IV. 40 CFR Part 372. Addition of Certain Chemicals; Toxic
Chemical Release Reporting; Community Right-to-Know; Proposed
Rule.
|
| June
14, 2001 - Implementation
of the Community Strategy for Endocrine Disruptors - a
range of substances suspected of interfering with the hormone
systems of humans and wildlife. Communication from the Commission
to the Council and the European Parliament. Commission of
the European Communities, Brussels COM (2001) 262 final. (More
information available at: http://europa.eu.int/comm/environment/docum/01262_en.htm
)
This
document presents a "priority list of substances for
further evaluation of their role in endocrine disruption.
During 2000, a candidate list of 553 man-made substances and
9 synthetic/natural hormones has been identified."
Organofluorine
pesticides in this list include: |
| Substances
included on the Endocrine Disruptor Liist |
CAS
No. |
Substances
included on the Endocrine Disruptor Liist |
CAS
No. |
| Bifenthrin |
82657-04-3
|
Flutriafol |
76674-21-0 |
| Cyhalothrin
(@Karate) |
91465-08-6 |
Fluvalinate |
69409-94-5 |
| Diflubenzuron |
35367-38-5 |
Prodiamine |
29091-21-2 |
| Epoxiconazole |
- |
Stannane,
tributylfluoro [Tributyltin fluoride] |
1983-10-4 |
| Fipronil |
- |
Thiazopyr |
- |
| Fluazifop-butyl |
69806-50-4
|
Trifluralin |
- |
| US
Federal Register |
| Published
Date |
Docket
Identification Number |
Details |
| August
18, 2004 |
OPP-2004-0054 |
Notice
of Receipt of Requests To Voluntarily Cancel Certain Pesticide
Registrations.
| It
is difficult to know if EPA included this as Fluvalinate
or Tau-fluvalinate. We have asked for clarification and
when we receive it we will amend it as necessary. |
| Registration
No. |
Product
Name |
EPA
Company No. |
Company
Name and Address |
| 002724
OR-99-0046 |
Mavrik
Aquaflow Insecticide |
000400 |
Wellmark
International,
1100 E.
Woodfield Rd., Suite 500,
Schaumbu, IL 60173. |
|
| Sept
13, 2002 |
OPP-2002-
0121 |
EPA
status of reregistration and tolerance reassessment. |
| July
31, 2002 |
OPP-2002-0155
|
Tolerance
Revocations. FINAL RULE.
With
the exception of honey, which is linked to the active registration
for use in/on beehives,
there are no active food-use registrations for the insecticide
fluvalinate. The use of fluvalinate on cotton was voluntarily
canceled in 1991. Cotton had been the only animal feed use for
fluvalinate; therefore, the animal commodity tolerances are
no longer needed. EPA believes that sufficient time has passed
for exhaustion of those stocks and for treated commodities to
have cleared channels of trade. Therefore, EPA is revoking the
tolerances in 40 CFR 180.427(a) for residues of fluvalinate
in or on cattle, fat; cattle, mbyp; cattle, meat; cottonseed;
cottonseed hulls; cottonseed oil (crude and refined); eggs;
goat, fat; goat, mbyp; goat, meat; hogs, fat; hogs, mbyp; hogs,
meat; horses, fat; horses, mbyp; horses, meat; milk; poultry,
fat; poultry, mbyp; poultry, meat; sheep, fat; sheep, mbyp;
and sheep, meat. Also,
a tolerance for coffee was established in 1989 based on a FIFRA
section 24(c) registration and use of fluvalinate on coffee
was restricted to Hawaii. In May 1990, the registration was
canceled. Therefore,
the Agency is revoking the tolerance in 40 CFR 180.427(c) for
residues of fluvalinate in or on coffee. |
| April
15, 2002 |
OPP-2002-0019 |
Proposed
Revocation of Tolerances - "With the exception of
honey, which is linked to the
active registration for use in/on beehives, there are no active
food-use registrations for the insecticide fluvalinate. The
use of fluvalinate on cotton was voluntarily canceled in 1991.
Cotton had been the only feed
use for fluvalinate; therefore, the animal commodity tolerances
are no longer needed. EPA believes that sufficient time has
passed for exhaustion of those stocks and for treated commodities
to have cleared channels of trade. Therefore, with the exception
of honey, EPA is proposing to revoke the tolerances in 40
CFR 180.427(a) for residues of fluvalinate in or on cattle,
fat; cattle, mbyp; cattle, meat; cottonseed; cottonseed hulls;
cottonseed oil (crude and refined); eggs; goat, fat; goat,
mbyp; goat, meat; hogs, fat; hogs, mbyp; hogs, meat; horses,
fat; horses, mbyp; horses, meat; milk; poultry, fat; poultry,
mbyp; poultry, meat; sheep, fat; sheep, mbyp; and sheep, meat.
Also, a tolerance for coffee was established in 1989 based
on a FIFRA section 24(c) registration and use of fluvalinate
on coffee was restricted to Hawaii. In May 1990, the registration
was canceled. Therefore, the Agency is proposing to revoke
the tolerance in 40 CFR 180.427(c) for residues of fluvalinate
in or on coffee.
Docket
control number OPP-2002-0019, |
| August
25, 1999 |
OPP-
34193 |
WELLMARK
- Request
to delete use from label of MARVIK AQUAFLOW INSECTICIDE: "commercial,
residential turf." |
| Oct
3, 1997 - Final |
na |
Cut
Roses. Exception Decisions to Early Entry Prohibition, Worker
Protection Standard; Technical Amendment.- FINAL RULE.
See also Federal Register of Oct 30, 1996 below. |
| Oct
3, 1997 - Proposed |
OPP-250121 |
Pesticide
Worker Protection Standard; Administrative Exception for Cut-Rose
Hand Harvesting; Administrative Decision. Proposed Rule. |
| August
4, 1997 |
OPP-300523 |
Pesticides
Subject to Tolerance Reassessment. |
| Oct
30, 1996 |
OPP-300164I |
Cut-Roses;
Request for Exception to Worker Protection Standard's Prohibition
of Early Entry into Pesticide-Treated Areas to Harvest Roses
by Hand Cutting. |
| May
15, 1996 |
OPP-34093 |
EPA's
Reregistration Eligibility Decision (RED) Development SCHEDULE. |
| Jan
12, 1994 |
OPPTS-400082 |
EPA's
proposal to add 41
fluorine and organofluorine chemicals to the Toxics Release
Inventory (TRI). See excerpt in box
above. Also available at http://www.epa.gov/tri/frnotices/59fr1788.htm |
|

