|
Return to Etoxazole
Adverse Effects
ACTIVITY:
Acaricide, Ovicide (unclassified)
CAS Name: 2-(2,6-difluorophenyl)-4-[4-(1,1-dimethylethyl)-2-ethoxyphenyl]-4,5-dihydrooxazole
Structure:
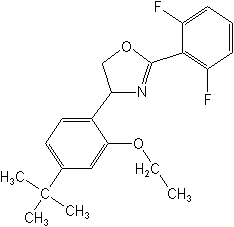
| Adverse
Effects:
Anemia
Body Weight Decrease
Bone
Endocrine: Adrenal
Endocrine: Prostate
Endocrine: Testicular
Genotoxic
Kidney
Liver
Mutagenicity
Teeth |
| Environmental
Effects:
Very
highly
toxic to aquatic invertebrates |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Yes
|
| US
EPA PC Code: |
107091 |
| US
Tolerances: |
CFR
180.593 |
Registered
use in
(includes only a limited list of countries)
|
France,
Israel, Japan, Korea, Taiwan, Turkey, South Africa, US |
| Japan's
Maximum Residue Levels (MRLs) |
Apple,
Beans (dry), Cherry, Cotton seeds, Cucumber, Eggplant, Grapefruit,
Hop, Lemon, Lime, Loquat, Melons, Natsudaidai (whole), Orange,
Other Citrus fruits, Peach, Pear, Strawberry, Tea, Tomato, Unshu
orange, Watermelon
-- Note
high level - 15 ppm - for Tea (Green, Black, Oolong, Wulung)
|
US
Maximum Residue Levels permitted
in food commodities
|
Tolerances
for 25 food commodities, including:
Apple, Cattle, Cotton, Goat, Horse,
Milk, Pear, Sheep, Strawberry, Tangerine
Existing
tolerances for Ornamental plants grown in greenhouses, shadehouses,
and lathhouses. |
| Other
Information |
| Molecular
Formula: |
C21H23
F2 NO2 |
| Manufacturers: |
Valent
Yashima Chemical Industry
Sumitomo Chemical |
| Other
Names: |
YI-5301,
TETRASAN,
Baroque, Virk, Zoom |
| Of
special interest: |
| PAN
Data |
| April
27, 2005 - U.S. Congresswoman Ellen Tauscher (D-CA) introduced
a bill "to suspend temporarily the duty on Etoxazole"
up to December 31, 2008. The bill was referred to the House
Committee on Ways and Means. |
| November
29, 2004. Review
report for the active substance etoxazole. European Commission.
SANCO/4054/2001 - rev. 3. |
| August
2003 - Summary
of Toxicology Data. California EPA. |
| August
2002 - Pesticide
Fact Sheet. US EPA. |
| 2001
- IR-4
New Products/Transitional Solution List - This
list contains brief descriptions of numerous new pest control
materials that have been introduced over the last several years.
Additionally, it contains information on some "older"
crop protection chemicals that are believed to have room for
new uses. This List includes Etoxazole |
| 2001
-
Glossary of Pesticide Chemicals.
A listing of pesticides subject to analysis
of residues in foods and feeds by the US Food and Drug Administration.
Also available at: http://vm.cfsan.fda.gov/~acrobat/pestglos.pdf
|
US
Federal Register
••
Note: The FR entries are too long to include at this
site.
Click here for
all entries. |
| Date
Published |
Docket
Identification Number |
Details |
| June 27, 2007 |
EPA-HQ-OPP-2007-0309 |
IR-4.
Pesticide
Petition. PP 6E7150. Proposal to establish a tolerance
for residues of the insecticide etoxazole in or on food commodities:
| hop, dried cones |
7.0 ppm |
melon, subgroup
9A
This subgroup includes
6 commodities.
cantaloupe • citron melon • melon •
melon, citron • muskmelon • watermelon
|
0.15 ppm |
| cherry |
0.70 ppm |
Practical analytical methods for detecting
and measuring levels of etoxazole have been developed and
validated in/on all appropriate agricultural commodities and
respective processing fractions. The LOQ of etoxazole in the
methods is 0.02 ppm which will allow monitoring of food with
residues at the levels proposed for the tolerances.
|
| July
20, 2005 |
OPP-2005-0170 |
VALENT.
Pesticide Tolerance.
FINAL RULE.
| Commodity
|
Parts
per million |
| Almond,
hulls |
2.0
ppm |
| Grape |
0.50
ppm |
| Grape,
raisin |
1.5
ppm |
Nut,
tree, group 14
This
group includes 16 commodities.
almond
• almond, hulls • beechnut • butternut
• cashew • chestnut • chinquapin
• filbert • nut, brazil • nut, hickory
• nut, macadamia • nutmeat, processed,
except peanut • nuts • pecan • pistachio
• walnut
|
0.01
ppm |
| Pistachio |
0.01
ppm |
A
summary of the toxicological endpoints for etoxazole used
for human risk assessment is discussed in Unit III.B. of the
final rule published in the Federal
Register of September 26, 2003
Excerpts
from the Sept. 26, 2003, Federal Register:
-- 90-Day oral toxicity rodents (rat).
NOAEL = 61.8/69.0 milligrams/kilogram/ day (mg/kg/day)
Male/Female (M/F) LOAEL = 183.7/204.8 mg/kg/day (M/F),
based upon increases in hepatic
enzyme levels, increased liver weights and centrilobular
hepatocellular swelling in both sexes and liver
enlargement in females only
-- 90-Day oral toxicity rodents
(mouse). NOAEL = 213.6/250.5
mg/kg/day (M/F) LOAEL = 878.4/994.5 mg/kg/day (M/F),
based upon periportal hepatocellular
necrosis, increased alkaline phosphatase levels,
accompanied by increased relative liver
weight, liver enlargement, and centrilobular hepatocellular
swelling
-- 90-Day oral toxicity nonrodents
(dog). NOAEL = 5.33/5.42
mg/ kg/day (M/F) LOAEL = 53.7/55.9 mg/ kg/day (M/F),
based upon clinical signs (vomiting foamy fluid and
mucous stool), clinical chemistry,
increased liver weights, and centrilobular swelling
in the liver and acinar
cell atrophy in the prostate.
-- Prenatal developmental toxicity in nonrodents (rabbit).
Maternal NOAEL = 200 mg/kg/day LOAEL = 1,000 mg/kg/day
based upon liver enlargement
and decreased body weight
gains and
food consumption. Developmental
NOAEL = 200 mg/kg/day LOAEL = 1,000 mg/kg/ day based
upon increased incidences
of 27 presacral vertebrae and 27 presacral vertebrae
with 13th ribs in the fetuses.
-- Reproduction and fertility
effects (rat). Parental/Systemic
NOAEL = 20 mg/kg/ day LOAEL = 100 mg/kg/ day (M/F),
based upon increased liver weights
in the P and F1 males and increased adrenal
weights in the P females Offspring/Systemic NOAEL
= 20 mg/kg/ day LOAEL = 100 mg/kg/ day (M/F), based
upon pup mortality Reproductive
NOAEL = 100 mg/kg/day LOAEL = not determined.
-- Combined chronic toxicity/ carcinogenicity rodents
(rat). NOAEL = 64 mg/kg/day
(M/F). LOAEL = not determined Equivocal
evidence of carcinogenicity.
-- 2-Year feed/ carcinogenic (rat)
. NOAEL = 1.83/2.07 mg/kg/day (M/F) LOAEL = 187/216
(M/ F), based upon effects on the incisors including
abnormal amelogenesis. No evidence
of carcinogenicity
-- Chronic toxicity nonrodents (dog).
NOAEL = 4.62/4.79 mg/kg/day (M/F). LOAEL = 23.5/23.8
mg/ kg/day (M/F), based upon increased alkaline phosphatase
activity, increased liver weights,
liver enlargement (females), and incidences of centrilobular
hepatocellular swelling in the liver.
-- 78-Week carcinogenic mouse. NOAEL = 242/243 (M/ F).
LOAEL = 484/482 (M/ F), based on a slight increase in
the incidence of a fatty change
in the centrilobular hepatocytes in males.
-- Gene mutation -
in vitro forward gene mutation assay in mouse lymphoma
cells. When tested up to cytotoxic levels, mutagenic
in the presence of S9 activation and equivocal
for mutagenicity in the absence of S9 activation.
-- Cancer. EPA has determined
that etoxazole is not likely to be a human carcinogen
and EPA therefore, does not expect it to pose a cancer
risk. As a result, a quantitative cancer dietary
exposure analysis was not performed. |
From the July 20, 2005, Federal Register.
• Prenatal and postnatal sensitivity.
There
is qualitative evidence of increased susceptibility following
exposure to etoxazole in the rat reproduction study.
Therefore, EPA performed a Degree of Concern Analysis to determine
the LOC for the effects observed when considered in the context
of all available toxicity data, and to identify any residual
uncertainties after establishing toxicity endpoints and traditional
UFs to be used in the risk assessment of this chemical. If
residual uncertainties are identified, EPA examines whether
these residual uncertainties can be addressed by a special
FQPA safety factor and, if so, the size of the factor needed.
In performing the Degree of Concern Analysis, EPA noted that
the effects in the pups in the rat reproduction study are
well-characterized with a clear NOAEL. In addition, the pup
effects occur at the same dose as maternal toxicity. Furthermore,
the doses selected for various risk assessment scenarios are
lower than the doses that caused off spring toxicity. There
are no residual uncertainties for prenatal/postnatal toxicity
in this study. Therefore, although there
is evidence of increased qualitative susceptibility in the
rat reproduction study, the concern is low. For the reasons
stated above, EPA has concluded that there is low concern
for prenatal and/or postnatal toxicity resulting from exposure
to etoxazole.
• EPA
determined that the 10X SF (safety factor) to protect infants
and children should be removed.
• Acute exposure... EPA
evaluated the suitability of the developmental
toxicity study in rabbits in which the developmental NOAEL
of 200 milligram/kilogram/day (mg/kg/day) is based upon increased
incidences of 27 presacral vertebrae and 27 presacral vertebrae
with 13th ribs (skeletal variations) in the fetuses at the
LOAEL of 1,000 mg/kg/day (limit dose). Although these
developmental effects may be attributed to a single dose,
EPA concluded that these effects are minor in magnitude and
were observed only at the limit dose (1,000 mg/kg/day).
Therefore,
quantitation of the acute risk was not performed.
|
| ••
Note: The FR entries are
too long to include at this site.
Click here for all entries.
|
|