|
Return to
Index Page
Abstracts
ACTIVITY: Acaricide,
Insecticide (organofluorine)
Note: Out-dated
pesticide
CAS Name: 2-fluoro-N-methyl-N-(1-naphthalenyl)acetamide
Structure:
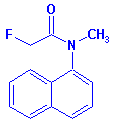
Adverse Effects:
Blood
Body Weight Decrease
Brain
Endocrine: Testes
Heart
Kidney
Liver
Lung
As
far as we know, this pesticide is not in use anymore. Very
little information available. Japan was one country where
there was considerable use. It was registered for tea growing.
|
Blood
(click
on for all fluorinated pesticides)
A health survey was carried out on 181 farmers before and after
the pesticidal application of Nissol (MNFA). Questionnaires on
general health, determination of blood pressure and body weight,
examination of urine and liver functions, physical examinations,
indirect chest roentgenography, blood picture, blood sugar level,
and ECG were recorded for some farmers.
Forty-five percent of the farmers complained of subjective symptoms
at the second examination (4 days after the application), unrelated
to the duration of working hours. The major subjective symptoms
were general malaise, headache, nausea, loss of appetite, diarrhea,
insomnia, abdominal pain, and tachypnea in that order. On the
average, body weight loss appeared in 33.6% of the farmers three
weeks after the application. Hemoglobin increased in 4.6%, decreased
in 18.7%, and did not change in 77% of the farmers.
Red cell count decreased in about 52% of the farmers. Leukocyte
count increased in less than fifty percent of the farmers with
a noticeable increase of neutrophils. Some
increase of urobilinogen and glycosuria were noticed while proteinuria
remained stable. Clinically
questionable findings were not obtained in liver functions. The
findings on ECG were a slight decrease of heart rate and a slight
elongation of PQ; however, no arrhythmia was found.
Ref: Studies of organofluorine pesticide
intoxication. II. Results of health examinations of farmers using
an organofluorine pesticide; by Hashida K. Okayama Igakkai Zasshi
(J. Okayama Med. So; 83(7-8): 295-310; 1971. [Abstract from Toxnet.]
Abstract. The selective toxicity of N-methyl-N- ( 1-naphthyl
) monofluoroacetamide ( MNFA ) in various species of animals and
the effects of the compound on the central action, the peripheral
action and the fluctuations in the cardiovascular and respiratory
systems were investigated. Tabulated data present the physiological
function or activity investigated, the test animal, the dosage
of MNFA administered and the route of administration. Results
showed that below the toxic level, MNFA had little or no general
pharmacologic effect and only a minute effect on the central and
peripheral nervous systems and various peripheral organs of the
differenct animals tested. When a toxic dose of MNFA was administered,
respiratory depression, a fall of blood pressure and body temperature
and a decrease in heart rate were generally observed.
Both the rat and cat developed convulsions.
Just prior to death, a flat wave was observed in the electrical
activity of the brain which was indicative of a serious impediment.
A drop in blood pressure
of about 30% was observed at 24 hr in rats that received 50 mg/kg
of MNFA orally. Cardiac response revealed
the characteristic feature of this compound to be cardiac depression
in every species tested. In addition,
among animals that have a high sensitivity to MNFA, such as the
guinea pig, dog and cat, bigeminal or trigeminal ventricular premature
beats were observed. An enhancement of epinephrine activity
by MNFA was also noted. MNFA had a slight effect on the red cell
count, but the white
cell count in rabbits decreased markedly accompanied by a decrease
of pseudoeosinophils and an increase of lymphocytes. The
blood sugar level in mice showed an initial increase prior to
a final decrease, while in rats and guinea pigs there was a decrease
and the value remained unchanged in rabbits and dogs. Ketone bodies
were only detected in the mouse.
Ref. Some pharmacologic properties
of a new fluorine pesticide, N-methyl- N- ( 1-Naphthyl ) monofluoroacetamide;
by Hasimoto Y, Noguchi T, Mori T, Kitagawa H. Toxicol. Appl. Pharmacol.;
13(2), 174-88, 1968.
Body
Weight Decrease
(click on for all fluorinated pesticides)
Abstract: A health survey was carried out on 181 farmers before
and after the pesticidal application of Nissol (MNFA). Questionnaires
on general health, determination of blood pressure and body weight,
examination of urine and liver functions, physical examinations,
indirect chest roentgenography, blood picture, blood sugar level,
and ECG were recorded for some farmers.
Forty-five percent of the farmers complained of subjective symptoms
at the second examination (4 days after the application), unrelated
to the duration of working hours. The major subjective symptoms
were general malaise, headache, nausea, loss of appetite, diarrhea,
insomnia, abdominal pain, and tachypnea in that order.
On the average, body weight loss appeared in 33.6% of the farmers
three weeks after the application. Hemoglobin increased
in 4.6%, decreased in 18.7%, and did not change in 77% of the
farmers. Red cell count decreased in about 52% of the farmers.
Leukocyte count increased in less than fifty percent of the farmers
with a noticeable increase of neutrophils.
Some increase of urobilinogen and glycosuria
were noticed while proteinuria remained stable. Clinically
questionable findings were not obtained in liver functions. The
findings on ECG were a slight decrease of heart rate and a slight
elongation of PQ; however, no arrhythmia was found.
Ref: Studies of organofluorine pesticide
intoxication. II. Results of health examinations of farmers using
an organofluorine pesticide; by Hashida K. Okayama Igakkai Zasshi
(J. Okayama Med. So; 83(7-8): 295-310; 1971. [Abstract from Toxnet.]
Brain
(click
on for all fluorinated pesticides)
Abstract.
The relationship between the characteristic electroencephalographic
findings and the blood sugar levels in Nissol (N-methyl-N (10naphthyl)
monofluoroacetamide) poisoning has been noted recently. States
of both acute and chronic intoxication by Nissol were produced
in male albino rabbits to study the compound's effects on the
brain, together with hematologic, renal and postmortem histological
manifestations. In the acute experiment, 2 ml of a 25% emulsion
was applied to the skin surface. In the chronic toxicity test,
a 1000-fold dilution of the 25% emulsion was applied to the skin
once daily until the animals succumbed. In the acute toxicity
test, there were no remarkable findings in the blood cell count,
hemoglobionmetry or livr function tests but the blood sugar decreased
from the pretreatment level of 122 to 78 and 56 mg/dl in 3 and
7 hr, respectively. In
the chronic toxicity test, the erythrocyte count decreased to
3,310,000 and hemoglobin dropped to 50% in 8 months.
The liver function tests, blood sugar analysis and electrocardiography
did not show any remarkable changes. The electroencephalogram
in the acute toxicity test exhibited a transient convulsive wave
in all leads 5 hr following the application of Nissol which then
gradually lapsed to a slow-voltage pattern, while the blood sugar
level dropped to 56 mg/dl. In the chronic experiment, the electroencephalographic
tracings showed a low-voltage pattern which fell into regular
waves in the eighth month without convulsions or a decrease in
blood sugar levels. I.P. injection of 20% glucose caused the temporary
development of an alpha wave. The visceral
organs were characterized by congestion, atrophy and degeneration
both in the chronic and the acute toxicity tests,
Ischemic changes in the cerebral cortex, hippocampus and Purkinje
cells of the cerebellum were more pronounced in the chronic experiment.
From
the above studies, it is concluded that there is a cause-effect
relationship between the fall of the blood sugar level, the electroencephalographic
findings and the ischemic changes in the brain in Nissol intoxication.
Ref:
Organic fluorine poisoning, by Nanba M JR et al. Nippon Rinsho;
29(2):864-70 1971. [Abstract from Toxnet.]
Abstract. The accumulation of citrate was studied in spider-mites,
house-flies and mice after treatment with the acaricide Nissol
(5903139). Male Swiss-Webster-mice were injected with various
concentrations of Nissol. House-flies were treated topically with
Nissol at various concentrations or received thoracic injections.
A slide/dip technique was used to dose two-spotted-spider mites
with Nissol. Mortality was recorded at 24 hours after treatment
and the median lethal dose (LD50) was calculated for each species.
The citric-acid (77929) content was determined in homogenates
of whole mice in brains, hearts, livers, and kidneys photospectrometrically.
Citric-acid content was also determined in homogenates of flies
and mites. The LD50 for intraperitoneal administration in mice
was 200 milligrams per kilogram (mg/kg). The topical LD50 in house-flies
was 525mg/kg and the injected LD50 was 14mg/kg. The LD50 for contact
administration to spider mites was 250 parts per million. Citric-acid
increased substantially in each species even by 3 hours after
dosing. The maximum accumulation in mice occurred at 6 hours.
Flies and mites continued to show increased accumulation through
12 hours. In the mouse citric-acid was accumulated
in decreasing order in the heart, kidney, brain, and liver. The
authors conclude that mites, flies and mice accumulate citrate
when treated with Nissol. The toxicity of
this acaricide may be related to inhibition of aconitase which
catalyzes transformation of citric-acid.
Ref. Citrate Accumulation In Twospotted
Spider Mites, House Flies, And Mice Following Treatment With The
Acaricide 2-Fluoro-N-methyl-N-(1-naphthyl) Acetamide; by Johannsen
FR. Knowles CO. Journal of Economic Entomology, Vol. 65, No. 6,
pages 1754-1756, 14 references, 1972.
Abstract. The effects of a single dose and repeated doses of
N-methyl-N- ( 1- naphthyl ) monofluoroacetamide (MNFA) on the
fluctuation of citrate in animals and the replationship between
the activity of MNFA hydrolysis and the acute toxicity of MNFA
in various species were investigated. MNFA
was administered intraperitoneally at 25 mg/kg to male Wistar
strain rats, 2.0 mg/kg to guinea pigs and 300 mg/kg to monkeys.
At specified periods after dosing, the animals were sacrificed
and the citrate content of heart, kidneys, liver and brain was
determined. For the multiple dose study, MNFA was administered
orally to male rats at doses of 0.625, 1.25, 2.5, 5.0 and 10.0
mg/kg/ day for 180 days and the citrate content was determined
in the brain, heart, liver, kidney, testis and blood.
In the rat, after a single dose of MNFA, the citrate level increased
to 27, 10,
10 and negligible times the
normal value in heart,
kidneys, brain and liver, respectively.
In the chronic toxicity experiment, the only increase
(3 times the control value) was in the testes of rats receiving
10 mg/ kg/day of MNFA. In all other
groups, the level in liver and kidney decreased significantly
in comparison with the levels in animals receiving a single dose.
It is suggested that this difference was due to metabolism and
to the detoxification mechanism of the liver
and kidney which may have been accelerated by the chronic administration
of MNFA. The citrate level in the monkeys after a single dose
was much lower than in the rat. In guinea pigs it
increased to the maximum at 9 hr when it reached 30 times the
control value in the kidney, 10 times in the heart,
6 times in the brain while no appreciable
increase was found in the liver. The hydrolysis of MNFA by liver
homogenates was closely related to the acute toxicity and the
product of the hydrolysis was determined as N-methyl-1-naphthylamine.
The enzyme activity in the guinea pig was about 35 times that
of the rat or mouse. The LD50 of MNFA was 3.1 times that
of N- ( 1-naphthyl ) monofluoroacetamide ( NFA ) and the amount
hydrolyzed after 30 min incubation was about one- fifth.
Ref: Studies of the biochemical lesions
caused by a new fluorine pesticide, N-methyl-N- ( 1-naphthyl )
monofluoroacetamide; by Noguchi T, Hashimoto Y, Miyata H. Toxicol.
Appl. Pharmacol.; 13(2), 189-98, 1968.
Abstract. The selective toxicity of N-methyl-N- ( 1-naphthyl
) monofluoroacetamide ( MNFA ) in various species of animals and
the effects of the compound on the central action, the peripheral
action and the fluctuations in the cardiovascular and respiratory
systems were investigated. Tabulated data present the physiological
function or activity investigated, the test animal, the dosage
of MNFA administered and the route of administration. Results
showed that below the toxic level, MNFA had little or no general
pharmacologic effect and only a minute effect on the central and
peripheral nervous systems and various peripheral organs of the
differenct animals tested. When a toxic dose of MNFA was administered,
respiratory depression, a fall of blood pressure and body temperature
and a decrease in heart rate were generally observed.
Both the rat and cat developed convulsions.
Just prior to death, a flat wave was observed
in the electrical activity of the brain which was indicative of
a serious impediment. A
drop in blood pressure of about 30% was observed at 24
hr in rats that received 50 mg/kg of MNFA orally.
Cardiac response revealed the characteristic feature of this compound
to be cardiac depression in every species tested. In
addition, among animals that have a high sensitivity to MNFA,
such as the guinea pig, dog and cat, bigeminal or trigeminal ventricular
premature beats were observed. An enhancement of epinephrine
activity by MNFA was also noted. MNFA had a slight effect on the
red cell count, but the white cell count
in rabbits decreased markedly accompanied by a decrease of pseudoeosinophils
and an increase of lymphocytes. The blood
sugar level in mice showed an initial increase prior to a final
decrease, while in rats and guinea pigs there was a decrease
and the value remained unchanged in rabbits and dogs. Ketone bodies
were only detected in the mouse.
Ref. Some pharmacologic properties
of a new fluorine pesticide, N-methyl- N- ( 1-Naphthyl ) monofluoroacetamide;
by Hasimoto Y, Noguchi T, Mori T, Kitagawa H. Toxicol. Appl. Pharmacol.;
13(2), 174-88, 1968.
TOXICITY
Ref: ChemIDplus for Nissol. Available
at Toxnet. |
Organism
|
Test
Type |
Route |
Reported
Dose (Normalized Dose) |
Effect |
Source |
| cat |
LD50 |
skin |
4mg/kg
(4 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
BEHAVIORAL:
EXCITEMENT |
Nippon
Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol.
65, Pg. 182, 1969.
Link
to PubMed |
| dog |
LD50 |
skin |
2750ug/kg
(2.75 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
BEHAVIORAL:
EXCITEMENT |
Nippon
Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol.
65, Pg. 182, 1969.
Link to PubMed |
| dog |
LDLo |
intraperitoneal |
2mg/kg
(2 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
BEHAVIORAL:
EXCITEMENT |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| guinea
pig |
LD50 |
skin |
5mg/kg
(5 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| guinea
pig |
LDLo |
subcutaneous |
1mg/kg
(1 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| monkey |
LDLo |
intraperitoneal |
100mg/kg
(100 mg/kg) |
BEHAVIORAL:
"HALLUCINATIONS, DISTORTED PERCEPTIONS"
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| monkey |
LDLo |
skin |
800mg/kg
(800 mg/kg) |
BEHAVIORAL:
"HALLUCINATIONS, DISTORTED PERCEPTIONS"
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| monkey |
LDLo |
subcutaneous |
150mg/kg
(150 mg/kg) |
BEHAVIORAL:
"HALLUCINATIONS, DISTORTED PERCEPTIONS"
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| mouse |
LD50 |
intraperitoneal |
164mg/kg
(164 mg/kg) |
BEHAVIORAL:
EXCITEMENT
BEHAVIORAL: COMA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| mouse |
LD50 |
subcutaneous |
216mg/kg
(216 mg/kg) |
BEHAVIORAL:
EXCITEMENT
BEHAVIORAL:
COMA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| rabbit |
LD50 |
oral |
1500ug/kg
(1.5 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Experimental
Animals. Jikken Dobutso Iho. Vol. 21, Pg. 88, 1972. |
| rabbit |
LDLo |
intravenous |
5mg/kg
(5 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| rat |
LD50 |
subcutaneous |
41mg/kg
(41 mg/kg) |
BEHAVIORAL:
EXCITEMENT
BEHAVIORAL: COMA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
Endocrine:
Testes
(click on for all fluorinated pesticides)
Abstract. The effects of a single dose and repeated doses of
N-methyl-N- ( 1- naphthyl ) monofluoroacetamide ( MNFA ) on the
fluctuation of citrate in animals and the replationship between
the activity of MNFA hydrolysis and the acute toxicity of MNFA
in various species were investigated. MNFA
was administered intraperitoneally at 25 mg/kg to male Wistar
strain rats, 2.0 mg/kg to guinea pigs and 300 mg/kg to monkeys.
At specified periods after dosing, the animals were sacrificed
and the citrate content of heart, kidneys, liver and brain was
determined. For the multiple dose study, MNFA was administered
orally to male rats at doses of 0.625, 1.25, 2.5, 5.0 and 10.0
mg/kg/ day for 180 days and the citrate content was determined
in the brain, heart, liver, kidney, testis and blood. In the rat,
after a single dose of MNFA, the citrate level increased to 27,
10, 10 and negligible times the normal value in heart, kidneys,
brain and liver, respectively. In
the chronic toxicity experiment, the only increase
(3 times the control value) was in the testes of rats
receiving 10 mg/ kg/day of MNFA. In
all other groups, the level in liver and kidney decreased significantly
in comparison with the levels in animals receiving a single dose.
It is suggested that this difference was due to metabolism and
to the detoxification mechanism of the liver
and kidney which may have been accelerated by the chronic administration
of MNFA. The citrate level in the monkeys after a single dose
was much lower than in the rat. In guinea pigs it
increased to the maximum at 9 hr when it reached 30 times the
control value in the kidney, 10 times in the heart, 6 times in
the brain while no appreciable increase was found in the liver.
The hydrolysis of MNFA by liver homogenates was closely related
to the acute toxicity and the product of the hydrolysis was determined
as N-methyl-1-naphthylamine. The enzyme activity in the guinea
pig was about 35 times that of the rat or mouse. The LD50 of MNFA
was 3.1 times that of N- ( 1-naphthyl ) monofluoroacetamide
( NFA ) and the amount hydrolyzed after 30 min incubation was
about one- fifth.
Ref: Studies of the biochemical lesions
caused by a new fluorine pesticide, N-methyl-N- ( 1-naphthyl )
monofluoroacetamide; by Noguchi T, Hashimoto Y, Miyata H. Toxicol.
Appl. Pharmacol.; 13(2), 189-98, 1968.
Abstract. The actue toxicity of
N-methyl-N- ( 1-napthyl ) fluoroacetamide ( MNFA, Nissol ) was
determined in detail in different species of animals and its subacute
toxicity was determined in the rat. The LD50 of Nissol and its
active ingredient MNFA were determined in male and female mice
by oral, subcutaneous, dermal, intraperitoneal and intravenous
administration; in male rats and guinea pigs by oral, dermal and
subcutaneous administration; in male rats and guinea pigs by oral,
dermal and subcutaneous administration; in male cats and dogs
by oral and dermal routes; in adult monkeys by oral, dermal, subcutaneous,
intraperitoneal and intravenous administration and orally in hens.
Fish toxicity was investigaged in carp and inhalation toxicity
tests were made in mice, rats and guinea pigs. The tabulated results
show that the toxicity varied markedly with
species. The toxicity of MNFA in mice, rats or monkeys
was 1/100 to 1/200 of that in rabbits, guinea pigs, cats or dogs.
In mice the oral LD50 ranged from 250-370 mg/kg, while by subcutaneous
and intraperitoneal administration, the LD50 values were about
250 mg/kg or less. The approximate toxicity of MNFA for monkeys
was less than for other species, the oral minimum lethal dose
( MLD ) being 300 mg/kg, subcutaneous MLD 150 mg/kg, intraperitoneal
MLD 100 mg/kg and dermal MLD 800 mg/kg.
The toxic symptoms produced by MNFA were charcteristic or organic
fluorine poisoning in mammals. In the subacute toxicity tests,
five groups of rats, 12 male and 12 female in each group were
given oral daily doses of 0.625, 1.25, 2.50, 5 and 10 mg/kg MNFA
for 6 months. Blood
and urine tests were taken at 40-day intervals and pathological
examination during autopsy revealed aspermatogenesis in some tubules
of the testis from the rats of the two high dose groups.
Destruction of the germinal epithelium was
more striking in mature cell series than in immature cells.
Does below 2.5 mg/kg did not produce significant changes in the
testis. A dose dependent decline in the
systolic arterial blood pressure was noticed. The reduction
ranged from 5 to 20% of the normal blood pressure. The blood pressure
reduction was not accompanied by gross or microscopic changes
of morphology of the cardiovascular system or kidney. The serum
level of SGOT also was reduced in the treated rats.
Ref. Acute an/ subchronic toxicity of a
new fluorine pesticide, N-methyl- N-( 1-naphthyl ) fluoracetamide;
by Hashimoto Y, Makita T, Miyata H, Noguchi T, Ohta G. Toxicol,
App. Pharmacol.; 12(3), 536-47, 1968.
Heart
(click
on for all fluorinated pesticides)
Abstract. The selective toxicity
of N-methyl-N- ( 1-naphthyl ) monofluoroacetamide ( MNFA ) in
various species of animals and the effects of the compound on
the central action, the peripheral action and the fluctuations
in the cardiovascular and respiratory systems were investigated.
Tabulated data present the physiological function or activity
investigated, the test animal, the dosage of MNFA administered
and the route of administration. Results showed that below the
toxic level, MNFA had little or no general pharmacologic effect
and only a minute effect on the central and peripheral nervous
systems and various peripheral organs of the differenct animals
tested. When a toxic dose of MNFA was administered, respiratory
depression, a fall of blood pressure and body temperature and
a decrease in heart rate were generally observed.
Both the rat and cat developed convulsions.
Just prior to death, a flat wave was observed in the electrical
activity of the brain which was indicative of a serious impediment.
A drop in blood pressure
of about 30% was observed at 24 hr in rats that received 50 mg/kg
of MNFA orally. Cardiac response revealed
the characteristic feature of this compound to be cardiac depression
in every species tested. In addition,
among animals that have a high sensitivity to MNFA, such as the
guinea pig, dog and cat, bigeminal or trigeminal ventricular premature
beats were observed. An enhancement of epinephrine activity
by MNFA was also noted. MNFA had a slight effect on the red cell
count, but the white cell count in rabbits
decreased markedly accompanied by a decrease of pseudoeosinophils
and an increase of lymphocytes. The blood sugar level in mice
showed an initial increase prior to a final decrease, while in
rats and guinea pigs there was a decrease and the value remained
unchanged in rabbits and dogs. Ketone bodies were only detected
in the mouse.
Ref. Some pharmacologic properties
of a new fluorine pesticide, N-methyl- N- ( 1-Naphthyl ) monofluoroacetamide;
by Hasimoto Y, Noguchi T, Mori T, Kitagawa H. Toxicol. Appl. Pharmacol.;
13(2), 174-88, 1968.
Abstract. General aspects of pesticide intoxication and therapy
have been reported previously. Since most pesticides act on the
nervous system, appreciable pathological alterations can be produced
there, especially in the CNS. Four cases of pesticide intoxication
are described: one with endrin, two with ceresin(!) (O-methyl
O-cyclohexyl S(p-chlorophenyl)thiophosphate), and one
with Nissol (MNFA)... In the MNFA intoxication case the patient's
condition was serious with unconsiousness and a very low blood
sugar value (50 mg/dl). The EEGs recorded on the first and seventh
days of hospitalization showed flat, low-voltage waves without
any slow waves or spikes. Intravenous administration of 40 ml
of 40% glucose solution restored the alpha-waves, although at
low-voltage, and the EEG returned to normal after one month. ...
Ref. Electroencephalograms in pesticide
poisoning cases; by Hiraki K, Iwasaki, Namba M. Rinsho Noha (Clin.
Electroencephalog.) ; 14(6): 333-340; 1972. [Abstract from Toxnet.].
Abstract. A brief discussion of the use of fluoroacetic acid
as a pesticide, and a brief history of studies on this acid are
given. More detailed discussions include those of MNFA, comparative
toxicity of MNFA, pre-clinical test of antidotes, fluoroacetic
poisoning of men and antidote treatment, the mechanism of detoxication,
and various experiments of fluoroacetic acid effects on the brains
of mice. The effects that have been detected
on men include: mild nausea, vomiting, headache, dizziness; medium
ataxia, clouding of consciousness, epileptic convulsion, repetition
of tonic and clonic convulsion, decrease
in heart rate; and serious coma, cyanosis,
lack of abdominal reflex, arrhythmia, increase of tracheal secretion,
and hypotension. Clinical findings include: sudden decrease or
increase of blood sugar, increase of hemogram leucocyte, increase
of pseudo acidophils, lymphocytes, and decrease of electrolyte
K. The blood pressure gradually decreases, and various
types of premature beats, myocardial infarction, and coronary
insufficiency are found on electrocardiogram, as the heart rate
increases and respiration decreases, In
hepatic function, GOT and GPT gradually or slightly increase.
Electroencephalogram shows a slow malfunction, or irregular
slow wave; when serious, a flat pattern appears. The
body temperature increases temporarily and then gradually decreases.
The article is a review
of previously published material by other researchers.
Ref. Pre-clinical evaluation of detoxication
of organic fluoride toxins; by Hashimoto Y. Eisei Kagaku (Journal
of Hygienic Chemistr; 17(6): 363-379; 1971. [Abstract from Toxnet.].
Abstract. The effects of N-methyl-N-(1-naphthyl)-monofluoroacetamide
(MNFA) on cardiac function were studied in rabbits. Rabbits received
5 milligrams per kilogram (mg/kg) MNFA orally, or 2mg/kg MNFA
subcutaneously (sc). Electrocardiographic (ECG) recordings were
made from subcutaneously implanted electrodes before and up to
2 hours after oral MNFA, and for up to 10 hours after sc MNFA.
Serum electrolytes and enzymes were determined 30 minutes before
and after, and 3 and 6 hours after MNFA. One hour after oral MNFA,
and ECG showed small deflections and almost discernible P-waves
and T-waves in lead 1, with tall peaked T-waves in leads 2, 3,
and aVF; after 2 hours, the QRS complex was widened and the ST
junction was lowered. Leads 1 and aVL had ST depression consisting
of wide S-waves and inverted T-waves. One hour after sc MNFA,
the ECG revealed tachycardia; after 3 hours the ST junction was
lowered. Leads 1 and aVL had ST depression consisting of wide
S-waves and inverted T-waves. One hour after sc MNFA, ECG revealed
tachycardia; after 3 hours the ST junction was lowered and T-waves
were flattened or inverted. After 6 hours, there were wide S-waves
and QRS was prolonged in leads 1, 2, and aVF. At 10 hours R-deflections
were decreased in most leads, and tachycardia and ST junction
depression were improved. Serum calcium was decreased slightly
6 hours after MNFA, but serum levels of sodium, potassium, chlorine,
transaminases, and lactic-dehydrogenase were not affected. The
authors conclude that the ECG changes of the QRS, ST, and T-waves
are probably caused mainly by metabolic disturbances during MNFA
intoxication.
Ref. Agricultural Organofluoride
Poisoning: II. Cardiac Damage; by Iwasaki I, Nawa H, Hara A, Takagi
S, Hyodo KFluoride, Vol. 3, No. 3, pages 127-130, 1970.
Abstract. Patients suffering from severe organofluoride intoxication
(MNFA, Oxylan) were treated with glucose.
Prior to treatment the electroencephalograms (EEG) demonstrated
flat curves. Dysrhythmic patterns were observed in less severely
affected patients which suggest the presence of impaired cerebral
function associated with abnormal carbohydrate metabolism. The
appearance of paroxysmal waves is also indicative
of organofluoride poisoning. It is
evident that EEG findings are valuable in the prognostic evaluation
and diagnosis of organofluoride poisoning.
Ref: Studies on organofluoride poisoning
IV. Electroencephalographic (EEG) observations; by Iwasaki I,
Namba, Nawa H, Hara, Tagaki S, Hyodo K. Fluoride; 3(3): 133-136;
1970.
Abstract. The accumulation of citrate was studied in spider-mites,
house-flies and mice after treatment with the acaricide Nissol
(5903139). Male Swiss-Webster-mice were injected with various
concentrations of Nissol. House-flies were treated topically with
Nissol at various concentrations or received thoracic injections.
A slide/dip technique was used to dose two-spotted-spider mites
with Nissol. Mortality was recorded at 24 hours after treatment
and the median lethal dose (LD50) was calculated for each species.
The citric-acid (77929) content was determined in homogenates
of whole mice in brains, hearts, livers, and kidneys photospectrometrically.
Citric-acid content was also determined in homogenates of flies
and mites. The LD50 for intraperitoneal administration in mice
was 200 milligrams per kilogram (mg/kg). The topical LD50 in house-flies
was 525mg/kg and the injected LD50 was 14mg/kg. The LD50 for contact
administration to spider mites was 250 parts per million. Citric-acid
increased substantially in each species even by 3 hours after
dosing. The maximum accumulation in mice occurred at 6 hours.
Flies and mites continued to show increased accumulation through
12 hours. In the mouse citric-acid was accumulated
in decreasing order in the heart, kidney, brain, and liver. The
authors conclude that mites, flies and mice accumulate citrate
when treated with Nissol. The toxicity of
this acaricide may be related to inhibition of aconitase which
catalyzes transformation of citric-acid.
Ref. Citrate Accumulation In Twospotted
Spider Mites, House Flies, And Mice Following Treatment With The
Acaricide 2-Fluoro-N-methyl-N-(1-naphthyl) Acetamide; by Johannsen
FR. Knowles CO. Journal of Economic Entomology, Vol. 65, No. 6,
pages 1754-1756, 14 references, 1972.
Abstract. The effects of a single dose and repeated doses of
N-methyl-N- ( 1- naphthyl ) monofluoroacetamide (MNFA) on the
fluctuation of citrate in animals and the replationship between
the activity of MNFA hydrolysis and the acute toxicity of MNFA
in various species were investigated. MNFA
was administered intraperitoneally at 25 mg/kg to male Wistar
strain rats, 2.0 mg/kg to guinea pigs and 300 mg/kg to monkeys.
At specified periods after dosing, the animals were sacrificed
and the citrate content of heart, kidneys, liver and brain was
determined. For the multiple dose study, MNFA was administered
orally to male rats at doses of 0.625, 1.25, 2.5, 5.0 and 10.0
mg/kg/ day for 180 days and the citrate content was determined
in the brain, heart, liver, kidney, testis and blood.
In the rat, after a single dose of MNFA, the citrate level increased
to 27, 10, 10 and negligible times
the normal value in heart, kidneys, brain
and liver, respectively. In
the chronic toxicity experiment, the only increase
(3 times the control value) was in the testes of rats receiving
10 mg/ kg/day of MNFA. In
all other groups, the level in liver and kidney decreased significantly
in comparison with the levels in animals receiving a single dose.
It is suggested that this difference was due to metabolism and
to the detoxification mechanism of the liver
and kidney which may have been accelerated by the chronic administration
of MNFA. The citrate level in the monkeys after a single dose
was much lower than in the rat. In guinea pigs it
increased to the maximum at 9 hr when it reached 30 times the
control value in the kidney, 10 times in
the heart, 6 times in the brain while no appreciable increase
was found in the liver. The hydrolysis of MNFA by liver homogenates
was closely related to the acute toxicity and the product of the
hydrolysis was determined as N-methyl-1-naphthylamine. The enzyme
activity in the guinea pig was about 35 times that of the rat
or mouse. The LD50 of MNFA was 3.1 times that of N- ( 1-naphthyl
) monofluoroacetamide ( NFA ) and the amount hydrolyzed after
30 min incubation was about one- fifth.
Ref: Studies of the biochemical lesions
caused by a new fluorine pesticide, N-methyl-N- ( 1-naphthyl )
monofluoroacetamide; by Noguchi T, Hashimoto Y, Miyata H. Toxicol.
Appl. Pharmacol.; 13(2), 189-98, 1968.
TOXICITY
Ref: ChemIDplus for Nissol. Available
at Toxnet. |
Organism
|
Test
Type |
Route |
Reported
Dose (Normalized Dose) |
Effect |
Source |
| dog |
LDLo |
intraperitoneal |
2mg/kg
(2 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
BEHAVIORAL:
EXCITEMENT
CARDIAC:
CARDIOMYOPATHY INCLUDING INFARCTION |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
Kidney
(click
on for all fluorinated pesticides)
Abstract. The accumulation of citrate was studied in spider-mites,
house-flies and mice after treatment with the acaricide Nissol
(5903139). Male Swiss-Webster-mice were injected with various
concentrations of Nissol. House-flies were treated topically with
Nissol at various concentrations or received thoracic injections.
A slide/dip technique was used to dose two-spotted-spider mites
with Nissol. Mortality was recorded at 24 hours after treatment
and the median lethal dose (LD50) was calculated for each species.
The citric-acid (77929) content was determined in homogenates
of whole mice in brains, hearts, livers, and kidneys photospectrometrically.
Citric-acid content was also determined in homogenates of flies
and mites. The LD50 for intraperitoneal administration in mice
was 200 milligrams per kilogram (mg/kg). The topical LD50 in house-flies
was 525mg/kg and the injected LD50 was 14mg/kg. The LD50 for contact
administration to spider mites was 250 parts per million. Citric-acid
increased substantially in each species even by 3 hours after
dosing. The maximum accumulation in mice occurred at 6 hours.
Flies and mites continued to show increased accumulation through
12 hours. In the mouse citric-acid was accumulated
in decreasing order in the heart, kidney, brain, and liver. The
authors conclude that mites, flies and mice accumulate citrate
when treated with Nissol. The toxicity of
this acaricide may be related to inhibition of aconitase which
catalyzes transformation of citric-acid.
Ref. Citrate Accumulation In Twospotted
Spider Mites, House Flies, And Mice Following Treatment With The
Acaricide 2-Fluoro-N-methyl-N-(1-naphthyl) Acetamide; by Johannsen
FR. Knowles CO. Journal of Economic Entomology, Vol. 65, No. 6,
pages 1754-1756, 14 references, 1972.
Abstract. The effects of a single dose and repeated doses of
N-methyl-N- ( 1- naphthyl ) monofluoroacetamide (MNFA) on the
fluctuation of citrate in animals and the replationship between
the activity of MNFA hydrolysis and the acute toxicity of MNFA
in various species were investigated. MNFA
was administered intraperitoneally at 25 mg/kg to male Wistar
strain rats, 2.0 mg/kg to guinea pigs and 300 mg/kg to monkeys.
At specified periods after dosing, the animals were sacrificed
and the citrate content of heart, kidneys, liver and brain was
determined. For the multiple dose study, MNFA was administered
orally to male rats at doses of 0.625, 1.25, 2.5, 5.0 and 10.0
mg/kg/ day for 180 days and the citrate content was determined
in the brain, heart, liver, kidney, testis and blood.
In the rat, after a single dose of MNFA, the citrate level increased
to 27, 10,
10 and negligible times the normal value in heart,
kidneys, brain and liver, respectively.
In the chronic toxicity experiment, the only increase
(3 times the control value) was in the testes of rats receiving
10 mg/ kg/day of MNFA. In all other
groups, the level in liver and kidney decreased significantly
in comparison with the levels in animals receiving a single dose.
It is suggested that this difference was due to metabolism and
to the detoxification mechanism of the liver
and kidney which may have been accelerated by the chronic administration
of MNFA. The citrate level in the monkeys after a single dose
was much lower than in the rat. In guinea pigs it
increased to the maximum at 9 hr when it reached 30 times the
control value in the kidney, 10 times in the heart,
6 times in the brain while no appreciable
increase was found in the liver. The hydrolysis of MNFA by liver
homogenates was closely related to the acute toxicity and
the product of the hydrolysis was determined as N-methyl-1-naphthylamine.
The enzyme activity in the guinea pig was about 35 times that
of the rat or mouse. The LD50 of MNFA was 3.1 times that
of N- ( 1-naphthyl ) monofluoroacetamide ( NFA ) and the amount
hydrolyzed after 30 min incubation was about one- fifth.
Ref: Studies of the biochemical lesions
caused by a new fluorine pesticide, N-methyl-N- ( 1-naphthyl )
monofluoroacetamide; by Noguchi T, Hashimoto Y, Miyata H. Toxicol.
Appl. Pharmacol.; 13(2), 189-98, 1968.
Liver
(click
on for all fluorinated pesticides)
Abstract. The effects of a single dose and repeated doses of
N-methyl-N- ( 1- naphthyl ) monofluoroacetamide (MNFA) on the
fluctuation of citrate in animals and the replationship between
the activity of MNFA hydrolysis and the acute toxicity of MNFA
in various species were investigated. MNFA
was administered intraperitoneally at 25 mg/kg to male Wistar
strain rats, 2.0 mg/kg to guinea pigs and 300 mg/kg to monkeys.
At specified periods after dosing, the animals were sacrificed
and the citrate content of heart, kidneys, liver and brain was
determined. For the multiple dose study, MNFA was administered
orally to male rats at doses of 0.625, 1.25, 2.5, 5.0 and 10.0
mg/kg/ day for 180 days and the citrate content was determined
in the brain, heart, liver, kidney, testis and blood.
In the rat, after a single dose of MNFA, the citrate level increased
to 27, 10, 10 and negligible times
the normal value in heart, kidneys, brain and liver, respectively.
In the chronic toxicity experiment, the only increase
(3 times the control value) was in the testes of rats receiving
10 mg/ kg/day of MNFA. In all other
groups, the level in liver and kidney decreased significantly
in comparison with the levels in animals receiving a single dose.
It is suggested that this difference was due to metabolism and
to the detoxification mechanism of the liver
and kidney which may have been accelerated by the chronic administration
of MNFA. The citrate level in the monkeys after a single dose
was much lower than in the rat. In guinea pigs it
increased to the maximum at 9 hr when it reached 30 times the
control value in the kidney, 10 times in the heart,
6 times in the brain while no appreciable increase was found in
the liver. The hydrolysis of MNFA by liver
homogenates was closely related to the acute toxicity and the
product of the hydrolysis was determined as N-methyl-1-naphthylamine.
The enzyme activity in the guinea pig was about 35 times that
of the rat or mouse. The LD50 of MNFA was 3.1 times that
of N- ( 1-naphthyl ) monofluoroacetamide ( NFA ) and the amount
hydrolyzed after 30 min incubation was about one- fifth.
Ref: Studies of the biochemical lesions
caused by a new fluorine pesticide, N-methyl-N- ( 1-naphthyl )
monofluoroacetamide; by Noguchi T, Hashimoto Y, Miyata H. Toxicol.
Appl. Pharmacol.; 13(2), 189-98, 1968.
Abstract. The accumulation
of citrate was studied in spider-mites, house-flies and mice after
treatment with the acaricide Nissol (5903139). Male Swiss-Webster-mice
were injected with various concentrations of Nissol. House-flies
were treated topically with Nissol at various concentrations or
received thoracic injections. A slide/dip technique was used to
dose two-spotted-spider mites with Nissol. Mortality was recorded
at 24 hours after treatment and the median lethal dose (LD50)
was calculated for each species. The citric-acid (77929) content
was determined in homogenates of whole mice in brains, hearts,
livers, and kidneys photospectrometrically. Citric-acid content
was also determined in homogenates of flies and mites. The LD50
for intraperitoneal administration in mice was 200 milligrams
per kilogram (mg/kg). The topical LD50 in house-flies was 525mg/kg
and the injected LD50 was 14mg/kg. The LD50 for contact administration
to spider mites was 250 parts per million. Citric-acid increased
substantially in each species even by 3 hours after dosing. The
maximum accumulation in mice occurred at 6 hours. Flies and mites
continued to show increased accumulation through 12 hours. In
the mouse citric-acid was accumulated in decreasing order in the
heart, kidney, brain, and liver. The authors conclude that mites,
flies and mice accumulate citrate when treated with Nissol. The
toxicity of this acaricide may be related to inhibition of aconitase
which catalyzes transformation of citric-acid.
Ref. Citrate Accumulation In Twospotted
Spider Mites, House Flies, And Mice Following Treatment With The
Acaricide 2-Fluoro-N-methyl-N-(1-naphthyl) Acetamide; by Johannsen
FR. Knowles CO. Journal of Economic Entomology, Vol. 65, No. 6,
pages 1754-1756, 14 references, 1972.
catalyzes transformation of citric-acid.
Ref. Citrate Accumulation In Twospotted
Spider Mites, House Flies, And Mice Following Treatment With The
Acaricide 2-Fluoro-N-methyl-N-(1-naphthyl) Acetamide; by Johannsen
FR. Knowles CO. Journal of Economic Entomology, Vol. 65, No. 6,
pages 1754-1756, 14 references, 1972.
Lung
(click
on for all fluorinated pesticides)
TOXICITY
Ref: ChemIDplus for Nissol. Available
at Toxnet. |
Organism
|
Test
Type |
Route |
Reported
Dose (Normalized Dose) |
Effect |
Source |
| guinea
pig |
LD50 |
skin |
5mg/kg
(5 mg/kg) |
LUNGS,
THORAX, OR RESPIRATION: DYSPNEA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| guinea
pig |
LDLo |
subcutaneous |
1mg/kg
(1 mg/kg) |
LUNGS,
THORAX, OR RESPIRATION: DYSPNEA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| mouse |
LD50 |
intraperitoneal |
164mg/kg
(164 mg/kg) |
LUNGS,
THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| mouse |
LD50 |
subcutaneous |
216mg/kg
(216 mg/kg) |
LUNGS,
THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| rabbit |
LD50 |
oral |
1500ug/kg
(1.5 mg/kg) |
LUNGS,
THORAX, OR RESPIRATION: OTHER CHANGES |
Experimental
Animals. Jikken Dobutso Iho. Vol. 21, Pg. 88, 1972. |
| rabbit |
LDLo |
intravenous |
5mg/kg
(5 mg/kg) |
LUNGS,
THORAX, OR RESPIRATION: DYSPNEA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| rat |
LD50 |
subcutaneous |
41mg/kg
(41 mg/kg) |
LUNGS,
THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
TOXICITY
Ref: ChemIDplus for Nissol. Available
at Toxnet. |
Organism
|
Test
Type |
Route |
Reported
Dose (Normalized Dose) |
Effect |
Source |
| cat |
LD50 |
oral |
2500ug/kg (2.5 mg/kg) |
- |
Experimental
Animals. Jikken Dobutso Iho. Vol. 21, Pg. 88, 1972. |
| cat |
LD50 |
skin |
4mg/kg
(4 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
BEHAVIORAL:
EXCITEMENT |
Nippon
Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol.
65, Pg. 182, 1969.
Link
to PubMed |
| chicken |
LDLo |
oral |
50mg/kg
(50 mg/kg) |
- |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
dog
|
LD50 |
oral |
2mg/kg
(2 mg/kg) |
- |
Experimental
Animals. Jikken Dobutso Iho. Vol. 21, Pg. 88, 1972. |
| dog |
LD50 |
skin |
2750ug/kg
(2.75 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
BEHAVIORAL:
EXCITEMENT |
Nippon
Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 65,
Pg. 182, 1969.
Link to PubMed |
| dog |
LDLo |
intraperitoneal |
2mg/kg
(2 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
BEHAVIORAL:
EXCITEMENT
CARDIAC:
CARDIOMYOPATHY INCLUDING INFARCTION |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
guinea
pig
|
LD50 |
oral |
500ug/kg
(0.5 mg/kg) |
- |
Experimental
Animals. Jikken Dobutso Iho. Vol. 21, Pg. 88, 1972. |
| guinea
pig |
LD50 |
skin |
5mg/kg
(5 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
LUNGS,
THORAX, OR RESPIRATION: DYSPNEA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| guinea
pig |
LDLo |
subcutaneous |
1mg/kg
(1 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
LUNGS,
THORAX, OR RESPIRATION: DYSPNEA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
monkey
|
LD50 |
oral |
300mg/kg
(300 mg/kg) |
- |
"Wirksubstanzen
der Pflanzenschutz und Schadlingsbekampfungsmittel," Perkow,
W., Berlin, Verlag Paul Parey, 1971-1976Vol. -, Pg. -, 1971/1976. |
| monkey |
LDLo |
intraperitoneal |
100mg/kg
(100 mg/kg) |
BEHAVIORAL:
"HALLUCINATIONS, DISTORTED PERCEPTIONS"
GASTROINTESTINAL:
NAUSEA OR VOMITING
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| monkey |
LDLo |
skin |
800mg/kg
(800 mg/kg) |
BEHAVIORAL:
"HALLUCINATIONS, DISTORTED PERCEPTIONS"
GASTROINTESTINAL:
NAUSEA OR VOMITING
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| monkey |
LDLo |
subcutaneous |
150mg/kg
(150 mg/kg) |
BEHAVIORAL:
"HALLUCINATIONS, DISTORTED PERCEPTIONS"
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
GASTROINTESTINAL:
NAUSEA OR VOMITING |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| mouse |
LD50 |
intraperitoneal |
164mg/kg
(164 mg/kg) |
BEHAVIORAL:
EXCITEMENT
BEHAVIORAL:
COMA
LUNGS,
THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| mouse |
LD50 |
oral |
200mg/kg
(200 mg/kg) |
- |
Oyo Yakuri.
Pharmacometrics. Vol. 4, Pg. 463, 1970. |
mouse
|
LD50 |
skin |
370mg/kg
(370 mg/kg) |
- |
Experimental
Animals. Jikken Dobutso Iho. Vol. 21, Pg. 88, 1972. |
| mouse |
LD50 |
subcutaneous |
216mg/kg
(216 mg/kg) |
BEHAVIORAL:
EXCITEMENT
BEHAVIORAL:
COMA
LUNGS,
THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
| rabbit |
LD50 |
oral |
1500ug/kg
(1.5 mg/kg) |
SENSE
ORGANS AND SPECIAL SENSES: MYDRIASIS (PUPILLARY DILATION):
EYE
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
LUNGS,
THORAX, OR RESPIRATION: OTHER CHANGES |
Experimental
Animals. Jikken Dobutso Iho. Vol. 21, Pg. 88, 1972. |
rabbit
|
LD50 |
skin |
1750ug/kg
(1.75 mg/kg) |
- |
Experimental
Animals. Jikken Dobutso Iho. Vol. 21, Pg. 88, 1972. |
| rabbit |
LDLo |
intravenous |
5mg/kg
(5 mg/kg) |
BEHAVIORAL:
CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD
LUNGS,
THORAX, OR RESPIRATION: DYSPNEA |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
rat
|
LD50 |
oral |
67mg/kg
(67 mg/kg) |
- |
Sangyo
Igaku. Japanese Journal of Industrial Health. Vol. 9, Pg. 563,
1967. |
| rat |
LD50 |
skin
|
213mg/kg
(213 mg/kg) |
- |
World Review
of Pest Control. Vol. 9, Pg. 119, 1970. |
| rat |
LD50 |
subcutaneous |
41mg/kg
(41 mg/kg) |
BEHAVIORAL:
EXCITEMENT
BEHAVIORAL:
COMA
LUNGS,
THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION |
Toxicology
and Applied Pharmacology. Vol. 12, Pg. 536, 1968. |
|

