|
Return to Diflufenican
Index Page
Activity: Herbicide
(anilide)
Structure:
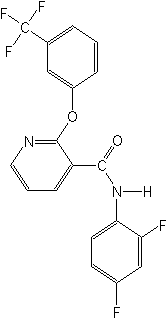
Adverse
Effects:
Body
Weight Decrease
Bone
Cholesterol
Endocrine: Testicular
Endocrine: Thymus
Liver
Environmental
| "Diflufenican/isoproturon"
ranked number 6 for "Most Widely
used pesticides in the UK (by Area Treated)"
| Rank
|
Formulation |
Method |
Area
treated (ha) |
Weight
applied (kg ai) |
| 6 |
Diflufenican/isoproturon |
Spray |
1,057,086 |
662,735 |
Ref:
April 29, 2000. UK Department for Environment, Food &
Rural Affairs in the online report, "Design of a Tax
or Charge Scheme for Pesticides. " Annex C3: Overview
of Pesticide Industry
http://www.fluorideaction.org/pesticides/pesticides.ranks.uk.2000.htm
|
Body
Weight Decrease
(click on for all fluorinated pesticides)
--Subacute toxicity
studies... In a 13-week oral study in the dog, the minimum effect
level was 250 mg/kg bw based on enemis and increased cholesterol
levels at the 250 mg/kg bw/day dose level. The observed effects
at higher dose levels included impaired
body weight gain, emesis, increased plasma cholesterol
and increase in the relative thymus weights.
-- Subchronic/chronic toxicity studies... The NOAEL in the 104-week
mouse study was 500 ppm (approximately 60 - 70 mg/kg bw/day) based
on minimal reduction in body weight gain at the 500 ppm dose level
and significant reduction in body weight
gain and increase in the absolute and relative
liver weight in females at the higher dose level. Diflufenican
was not tumourigenic in the mouse. The NOEL in the 52-week oral
study in the dog was 100 mg/kg bw/day based on significant increase
in liver weight at the higher dose
level.
-- Reproductive toxicity studies. In a teratogenicity study in
the rat, the minimum effect level for maternal toxicity was 50
mg/kg bw/day based on reductions in weight
gain at all dose levels...
-- Acceptable daily intake (ADI). The acceptable daily intake
of 0.2 mg/kg bw/day is derived from the critial minimum effect
level of 500 ppm (24 mg/kg bw/day), derived from the 24-month
chronic dietary study in the rat or the multigeneration study
in the rat and allows for a 100-fold safety factor. The observed
effects included minimal reductions in body
weight gain and a slight reduction in
thymus weights at the 500 ppm dose level.
Ref: September 1995. Evaluation
on Diflufenican. Evaluation of fully approved or provisionally
approved products. Department for Environment, Food and Rural
Affairs, Pesticides Safety Directorate, Mallard House, Kings Pool,
3 Peasholme Green, York YO1 7PX, UK. Available at:
http://www.pesticides.gov.uk/citizen/evaluations/evallist_alphabet.htm
Bone
(click
on for all fluorinated pesticides)
-- Reproductive toxicity
studies. In a teratogenicity study in the rat, the minimum effect
level for maternal toxicity was 50 mg/kg bw/day based on reductions
in weight gain at all dose levels. The NOEL for foetotoxicity
and teratogenicity was 5000 mg/kg bw/day. In a teratogenicity
study in the rabbit, the NOAEL for maternal toxicity was 50 mg/kg
bw/day based on clinical signs of toxicity and the NOEl for foetotoxicity
and teratogenicity 2500 mg/kg bw/day. A dose-related
increase in the incidence of supernumerary ribs was considered
to be within the historical control range. It was noted that the
incidence in controls was low, whilst the statistical significance
was marginal at the highest dose level.
Ref: September
1995. Evaluation on Diflufenican.
Evaluation of fully approved or provisionally approved products.
Department for Environment, Food and Rural Affairs, Pesticides
Safety Directorate, Mallard House, Kings Pool, 3 Peasholme Green,
York YO1 7PX, UK. Available at:
http://www.pesticides.gov.uk/citizen/evaluations/evallist_alphabet.htm
Cholesterol
(click
on for all fluorinated pesticides)
--Subacute toxicity studies... In a 13-week oral study in the
dog, the minimum effect level was 250 mg/kg bw based on enemis
and increased cholesterol levels
at the 250 mg/kg bw/day dose level. The observed effects at higher
dose levels included impaired body weight gain, emesis, increased
plasma cholesterol and increase in
the relative thymus weights.
Ref: September 1995. Evaluation
on Diflufenican. Evaluation of fully approved or provisionally
approved products. Department for Environment, Food and Rural
Affairs, Pesticides Safety Directorate, Mallard House, Kings Pool,
3 Peasholme Green, York YO1 7PX, UK. Available at:
http://www.pesticides.gov.uk/citizen/evaluations/evallist_alphabet.htm
Endocrine:
Testicular
(click
on for all fluorinated pesticides)
-- Subchronic and chronic toxicity studies. Rat. ... In a 24-month
feeding study, groups of 50 male and 50 female F344 rats (main
study groups, except the control group which consisted of 85 males
and 85 females) were administered diflufenican (98.4% purity)
in the diet, at concentrations of 0 (control), 500, 2500 or 12500
ppm. A further 30 male and 30 female rats per dose group (satellite
study) were similarly treated to generate toxicity dta. An interim-kill
was performed on 10 males and 10 females per dose group of the
satellite study after 52-weeks... The organ weight changes noted
after 104 weeks in the main group... There was an increase in
the incidence of marked/severe degeneration
of the tubular geminal epithelium of the testes at dose
levels of 500 ppm (32/37), 2500 ppm (31/32) and 12500 (33/39)
compared to controls (44/64). However, the incidence of slight/moderate
degeneration of the geminal epithelium was greater in controls
(20.64) compared to top dose males (6/39). Testicular geminal
tubular degeneration was considered to be related to pressure
atrophy arising from the high incidence of benign intestitial
cell tumours (75 - 90%) in treated and control animals...
Ref: September 1995. Evaluation
on Diflufenican. Evaluation of fully approved or provisionally
approved products. Department for Environment, Food and Rural
Affairs, Pesticides Safety Directorate, Mallard House, Kings Pool,
3 Peasholme Green, York YO1 7PX, UK. Available at:
http://www.pesticides.gov.uk/citizen/evaluations/evallist_alphabet.htm
Endocrine:
Thymus
(click
on for all fluorinated pesticides)
--Subacute toxicity studies... In a 13-week oral study in the
dog, the minimum effect level was 250 mg/kg bw based on enemis
and increased cholesterol levels at the
250 mg/kg bw/day dose level. The observed effects at higher dose
levels included impaired body weight gain, emesis, increased
plasma cholesterol and increase in the relative thymus
weights.
-- Acceptable daily intake (ADI). The acceptable daily intake
of 0.2 mg/kg bw/day is derived from the critial minimum effect
level of 500 ppm (24 mg/kg bw/day), derived from the 24-month
chronic dietary study in the rat or the multigeneration study
in the rat and allows for a 100-fold safety factor. The observed
effects included minimal reductions in body weight gain and a
slight reduction in thymus weights
at the 500 ppm dose level.
Ref: September 1995. Evaluation
on Diflufenican. Evaluation of fully approved or provisionally
approved products. Department for Environment, Food and Rural
Affairs, Pesticides Safety Directorate, Mallard House, Kings Pool,
3 Peasholme Green, York YO1 7PX, UK.at:
http://www.pesticides.gov.uk/citizen/evaluations/evallist_alphabet.htm
Liver
(click
on for all fluorinated pesticides)
-- Subchronic/chronic
toxicity studies... The NOAEL in the 104-week mouse study was
500 ppm (approximately 60 - 70 mg/kg bw/day) based on minimal
reduction in body weight gain at the 500 ppm dose level and significant
reduction in body weight gain and increase in the absolute and
relative liver weight in females
at the higher dose level. Diflufenican was not tumourigenic in
the mouse. The NOEL in the 52-week oral study in the dog was 100
mg/kg bw/day based on significant increase
in liver weight at the higher dose level.
Ref: September 1995. Evaluation
on Diflufenican. Evaluation of fully approved or provisionally
approved products. Department for Environment, Food and Rural
Affairs, Pesticides Safety Directorate, Mallard House, Kings Pool,
3 Peasholme Green, York YO1 7PX, UK. Available at:
http://www.pesticides.gov.uk/citizen/evaluations/evallist_alphabet.htm
|
Environmental
(click
on for all fluorinated pesticides)
--
Risk to mammals. Since new environmental fate and behaviour
data have reported that diflufenican
persists in the soil, there may be an increased risk
of secondary poisoning to small mammals feeding on contaminated
earthworms. Therefore, the risk of secondary poisoning to
small earthworm eating mammals will be addressed using the
available mammalian toxicity data.
... highly persistent in the soil
with dissipation DT50 values of 311 to 733 days,
as well as in the water body
with little hydrolysis over a range of pH levels at 22C.
This could lead to prolonged contamination of water due
to run-off of soil containing residues and through direct
contamination of water by overspray or spray drift (page
43).
Ref: September
1995. Evaluation
on Diflufenican. Evaluation of fully approved or provisionally
approved products. Department for Environment, Food and
Rural Affairs, Pesticides Safety Directorate, Mallard House,
Kings Pool, 3 Peasholme Green, York YO1 7PX, UK. Available
at: http://www.pesticides.gov.uk/citizen/evaluations/evallist_alphabet.htm
|
Note
from FAN:
The following reports can be purchased
from NTIS (National Technical Information Service) via phone
at 703-605-6000. The cost for each report (generally on
fiche) is approximately $25.00. If you have a copy of any
of these reports please share them with us. - Thanks, EC.
|
Source:
EPA/OTS; Doc #88-920009606
INITIAL
SUBMISSION: LETTER FROM RHONE-POULENC INC TO USEPA REGARDING
A ONCOGENICITY AND TOXICITY STUDY OF DIFLUFENICAN IN MICE
WITH ATTACHMENTS AND COVER LETTER DATED
08-31-92
Corporate
Name: LIFE SCI RESEARCH
Keywords:
RHONE-POULENC INC
DIFLUFENICAN ; HEALTH EFFECTS ; CHRONIC TOXICITY ;
CARCINOGENICITY ; MAMMALS ; MICE ; ORAL ; DIET
Order
Number: NTIS/OTS0571263
|
Source:
EPA/OTS; Doc #88-920009402
INITIAL
SUBMISSION: TERATOLOGICAL STUDY OF N-(2,4-DIFLUOROPHENYL)-2-[3-(TRIFLUOROMETHYL)PHENOXY]-3-PYRIDINECARBOXAMIDE
IN RABBITS WITH COVER LETTER DATED
08/31/92
Keywords:
RHONE-POULENC INC ; [Diflufenican] ; HEALTH EFFECTS ; REPRODUCTION/FERTILITY
EFFECTS ; TERATOGENICITY ; MAMMALS ; RABBITS ; ORAL ; GAVAGE
; SUBCHRONIC TOXICITY
Order
Number: NTIS/OTS0555455
|
Source:
EPA/OTS; Doc #88-920009414
INITIAL
SUBMISSION: EFFECT OF N-(2,4-DIFLUOROPHENYL)-2-[3-(TRIFLUOROMETHYL)PHENOXY]-3-PYRIDINECARBOXAMIDE
IN PREGNANT RATS WITH COVER LETTER DATED
08/31/92
Corporate
Name: HUNTINGDON RESEARCH CTR
Keywords:
RHONE-POULENC INC [Diflufenican] ; HEALTH EFFECTS ; REPRODUCTION/FERTILITY
EFFECTS ; TERATOGENICITY ; MAMMALS ; RATS ; ORAL ; GAVAGE
; SUBCHRONIC TOXICITY
Order
Number: NTIS/OTS0555467
|
Source:
EPA/OTS; Doc #88-920009450
INITIAL
SUBMISSION: COMBINED ONCOGENICITY AND TOXICITY STUDY OF
N-(2,4-DIFLUOROPHENYL)-2-[3-(TRIFLUOROMETHYL)PHENOXY]-3-PYRIDINE-CARBOXIMIDE]
IN RATS WITH COVER LETTER DATED
08-31-92
Source:
EPA/OTS; Doc #88-920009450
Keywords:
RHONE-POULENC INC ; [Diflufenican] ; HEALTH EFFECTS ; CHRONIC
TOXICITY ; COMBINED CHRONIC TOXICITY/CARCINOGENICITY ; MAMMALS
; RATS ; ORAL ; DIET
Order
Number: NTIS/OTS0571106
|
Source:
EPA/OTS; Doc #88-920008945
INITIAL
SUBMISSION: EFFECT OF M&B 38544 ON REPRODUCTIVE FUNCTION
OF MULTIPLE GENERATIONS IN THE RAT WITH COVER LETTER DATED
08/31/92
Corporate
Name: HUNTINGDON RESEARCH CENTRE LTD
Keywords:
RHONE-POULENC INC ; M&G 38544 ; HEALTH EFFECTS ; REPRODUCTION/FERTILITY
EFFECTS ; COMBINED TERATOGENICITY/REPRODUCTIVE EFFECTS ;
MAMMALS ; RATS ; ORAL ; DIET ; CHRONIC TOXICITY ; COMBINED
CHRONIC TOXICITY/CARCINOGENICITY
Order
Number: NTIS/OTS0555262
|
|

