|
Return
to
Adverse
Effects
Abstracts
ACTIVITY: Insecticide,
Wood preservative, US EPA List 4B Inert
Inorganic
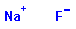
| Adverse
Effects: |
| The
following
are listed below
in this
section of Adverse Effects: |
Due
to length,
the following are presented
as separate sections |
| Cancer
Chemical Weapon
Precursor
CNS
Diabetes
Endocrine: Breast
Endocrine: Hypothalamus
Endocrine: Ovary
Endocrine: Uterus
Heart
Mesenteric artery
Pancreas
Salivary glands
Spleen
Teratogen
|
Apoptosis
Blood
Bone
Brain
Clastogenic
• Cytotoxic • Fetotoxic • Genotoxic
or Mutagenic
Endocrine: Pineal
Gland
Endocrine: Testicular
G-Proteins
I.Q.
Kidney
Liver
Lung
Reproductive
|
| Environmental
effects:
In
1979-1980 the upstream migration of adult spring chinook salmon
in the Columbia River were subject to unusally long delays.
Investigators found high concentrations of fluoride in the
vicinity of John Day Dam (0.3-0.5 mg/L in 1982) from discharges
from an aluminum factory. Experiments
concluded that the behavior of upstream-migrating adult salmon
would be adversely affected by fluoride concentrations of
about 0.5 mg/L. |
Fine:
Feb 19, 1999: The US Commerce Department's Export Administration
imposed a civil penalty of $750,000 on ALCOA for 100 violations
of US export regulations involving shipments of potassium fluoride
and
sodium fluoride. Potassium fluoride
and sodium fluoride are controlled
because they can be used to make chemical weapons. |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Yes |
| US
EPA PC Code: |
075202
|
| California
Chemical Code |
537
|
Registered
use in
(includes only a limited list of countries)
|
Australia,
Canada, New Zealand, US, Vietnam |
| Other
Information |
| Molecular
Formula: |
F
Na
Inorganic |
| Manufacturers: |
ALCOA
and other aluminum industries |
Other
Names:
--
too many to list here |
Alcoa
Sodium Fluoride
Antibulit
Floridine
Fluorol
Flursol
FDA 0101
NCI-C55221
Pergantene Roach Salt
Sodium Hydrofluoride
Sodium Monofluoride
ADZ-PAD
Villiaumite
Hollow Heart Concentrate
Tie-Gard
Florocid
Tie-Gard |
| Of
special interest: |
| PAN
BAD ACTOR - Acute Toxicity |
| TOXNET
Profile from the Hazardous Substances Data Bank -
This profile has been updated and is available at Toxnet.
|
| December 10, 2007: Comments on EPA's Assessment for Wood Treatment Products from Fluoride Action Network. |
August
1, 2005. Comments submitted
to EPA on its proposal to revoke "tolerance exemptions"
for sodium fluoride as an inert ingredien by FAN Pesticide
Project. Federal Register Docket OPP-2005-0069.
Note: Currently sodium fluroide is classified as an EPA List
4B inert. All List 4 Inerts are approved for use in the
US National Organic Program. If EPA approves a Final Rule to
revoke "tolerance exemptions," sodium fluoride will
be removed from the "inerts" list. This is good news. |
2005
- Schedule for Reregistration & Tolerance Reassessment (RED)
is expected to be August 2008. Contact at EPA: Rebecca Miller
(703) 305-0012; miller.rebecca@epa.gov . According to EPA:
Through the pesticide reregistration and tolerance reassessment
programs, EPA is assessing risks and making risk management
decisions for older pesticides. These decisions are summarized
in documents known as REDs, IREDs, and TREDs. By making decisions
according to the schedule below, EPA will meet its statutory
deadlines for completing reregistration and tolerance reassessment.
Some of the decision dates presented in the schedule may change
due to the dynamic nature of the review process. Any pesticide
decisions that are not completed during the current fiscal year
will be rescheduled for the following year. EPA is committed
to meeting its reregistration and tolerance reassessment deadlines.
http://www.epa.gov/pesticides/reregistration/decision_schedule.htm |
| May 3, 2004. Submission
to: National Research Council Committee: Toxicologic Risk
of Fluoride in Drinking Water. From Ellen Connett. Title of
Submission: Fluoride's adverse effects
on the Male Reproductive system |
| April 19, 2004.
Submission
to National Research Council Committee: Toxicologic Risk
of Fluoride in Drinking Water. From Ellen Connett. Title of
Submission: Fluoride's effect on the brain. |
2001
-
US
EPA "List 4 Inerts".
Uses: Not more than 0.25% of pesticide
formulation. Stabilizer carrier for formulations used before
crop emerges from soil. Ref: http://www.fintrac.com/gain/traderegs/usa/40P0180D.pdf |
| Material
Safety Data Sheet. Sigma Chemical Co. |
| Pesticide
Products - partial list |
| June
10, 2002 -
European Directive
on Food Supplements: Included for
use are Fluoride, Potassium
fluoride, and Sodium
Fluoride. European Parliamment
and the Council of the European Union. |
| February
19, 1999 -
ALCOA Fined $750,000
by Commerce Department For Illegal Chemical Shipments -
US Dept. of Commerce, Bureau of Export Administration --see
also at: http://www.bxa.doc.gov/press/99/alcoa.htm |
| October
1998 - Structural
Pest Management pesticides. FAN's
compilation of information cited on fluorine and organofluorine
pesticides published in General Pest Management, Category 7A.
A Guide for Commercial Applicators. Prepared by: Carolyn Randall,
MSU Pesticide Education Program. Published by Michigan
State University; MSU
manual number: E-2048. |
| March
1998 - Hazardous
Substance Fact Sheet - NJ Department
of Health and Senior Services |
April
9, 2001. Australia. Exemptions Listing
TECHNICAL GRADE ACTIVE
CONSTITUENTS EXCLUDED FROM THE REQUIREMENTS OF NRA APPROVAL
The list generally includes chemicals
which have not been primarily developed as agricultural chemicals
and thus for which an extensive package of data would not be
readily available. Approval by the National Registration Authority
for these TGACs is currently not required. Fluoride
compounds exempted
include: Cupro-ammonium Fluoroborate complex, Sodium
fluoride, Sodium fluoroacetate,
Sodium fluorosilicate. |
| Abstracts
mainly from PubMed and TOXNET. Sodium fluoride is the substance
of choice in animal studies to determine fluoride's adverse
effects. Because of the number of studies performed, these abstracts,
mainly dealing with Sodium fluoride, are listed by year. |
| 1999
-
Recognition and Management of
Pesticide Poisonings: Fluorides -
US EPA Office of Pesticide Programs |
| December
2000 - Sodium
Fluoride Proposed for Inclusion in National Organic Standards
|
National
Toxicology Program.
Drinking Water Study, number TR-393.
These studies, conducted by Battlelle
Columbus Laboratory in Ohio, are
steeped in controversy. The union that represents EPA professionals
in Washington DC have requested, in Congressional testimony,
that these studies be redone. For more information search
FAN's website at http://www.fluoridealert.org
Toxicology & Carcinogenesis
study of rats and mice - 15 pages.
Toxicology & Carcinogenesis
study of rats and mice - shorter report
Toxicology & Carcinogenesis
study of rats and mice - Tables |
| Sodium
Fluoride: A Classified Hazardous Waste California
Code of Regulations, Title 22, Section 66261.126 |
Sodium
fluoride is a crystalline mineral once widely used in the
United States for control of larvae and crawling insects
in homes, barns, warehouses, and other storage areas. It
is highly toxic to all plant and animal life. The
only remaining use permitted is for wood treatement.
Ref: Recognition and
Management of Pesticide Poisonings,
5th Edition,
The Office of Pesticide Programs, US EPA. Chapter 8
http://www.epa.gov/oppfead1/safety/healthcare/handbook/Chap08.pdf
Note
from FAN:
Unfortunately the above is
not correct. Sodium fluoride is allowed for use
on food crops as a US EPA "List 4 Inert."
"Inerts" are treated as confidential
proprietary information by US EPA and the public
is denied the right to know which pesticides contain
them or on what food crops they are used. All "List
4 Inerts" (which includes Sodium fluoride)
were approved for use in the US
National Organic Program - (see last paragraph
on page 248).
For
more information on the issue of "Inerts"
see: Toxic
Secrets: "Inert" Ingredients in Pesticides1987-1997,
published by Northwest Coalition for Alternatives
to Pesticides.
|
Sodium
fluoride is currently registered for use as a wood preservtive
in Canada. In 2000, 12 tonnes of NaF were used for this
purpose. Nearly all of this amount
came from sales of a wood preservative paste applied to
the groundline portion of in-service utility poles. The
only registration for sodium fluoride as an insecticide
expired December 2000 (p 4).
Ref: Canadian
Water Quality Guidelines for the Protection of Aquatic Life:
Inorganic Fluorides.
Environment Canada. August
2001.
|
US
EPA List of Inerts. This
substance is on List 4B
EPA's
List 4A & 4B Inerts have been approved for use in the
new US National Organic Standards. Sodium Fluoride as a
List 4B Inert is allowed for use in organic agriculture
in the US.
Note:
US EPA
allows so-called "Inert" ingredients to be commonly mixed
with the "active" pesticidal ingredient to create
a formulated pesticide product. According
to EPA, "The term `inert' is not intended to imply
nontoxicity; the ingredient may or may not be chemically
active." "Inert" ingredients include solvents,
emulsifiers, spreaders, and other substances mixed into
pesticide products to increase the effectiveness of the
active ingredients, make the product easier to apply, or
to allow several active ingredients to mix in one solution.
Both US EPA and California Department of Pesticide Regulation
require pesticide manufacturers to identify inert ingredients
in their products but do not disclose this information to
the general public because the pesticide industry considers
product formulations trade secrets, protected by law and
by the US EPA. The US EPA category of Inerts (as of September
2003):
List 1 - Of Toxicological
Concern
List 2 - Potentially
Toxic / High Priority for Testing
List
3 - Of Unknown Toxicity
List 4A - Generally Regarded as Safe
List 4B - EPA states it has
Sufficient Information to Reasonably Conclude that the Current
Use Pattern in Pesticide Products will not Adversely Affect
Public Health or the Environment
List
4 (all)
|
*USES:
This chemical is used as an insecticide, a constituent of
vitreous enamel and glass mixtures and as a steel degassing
agent. It is used in electroplating fluxes, heat treating
salt compositions and fluoridation of drinking water. It
is also used as a disinfectant for brewery apparatus,
for preserving wood,
pastes and mucilage, in the manufacture of coated paper
in frosting glass and in the removal of hydrogen fluoride
from exhuast gases. It is used as a dental caries prophylactic.
In veterinary medicine, it is used as an anthelmintic
[an agent that destroys or expels intestional worms], a
pediculicide [an agent used
to destroy lice] and an acaricide
[commonly used to denote chemicals that kill ticks].
It is also used as a preservative
for toothpastes, in laundry soap and in
cryolite manufacture. Single crystals are used as
windows in ultraviolet and infrared radiation detecting
devices.
*ACUTE/CHRONIC HAZARDS: This compound is toxic by ingestion,
inhalation and skin contact. It may cause irritation. When
heated to decomposition it emits toxic fumes of
hydrogen fluoride gas
and sodium oxide. It is corrosive.
Ref: National Toxicology
Program, Health and Safey, H&S: SODIUM FLUORIDE 7681-49-4
(updated on 13 August 2001. Available at:
http://ntp-server.niehs.nih.gov/cgi/iH_Indexes/ALL_SRCH/iH_ALL_SRCH_Frames.html
[Note: definitions from Stedman's Concise Medical Dictionary
for the Health Professions, 4th edition.]
|
Fluoride/fluorinated
substances identified in Agreement between Canada and the
United States on Great Lakes Water Quality, 1978.
Appendix 1 -
Hazardous Polluting Substances:
Ammonium Bifluoride * Ammonium Fluoborate * Ammonium Fluoride
* Ammonium Silicofluoride * Antimony Trifluoride * Beryllium
Fluoride * Ferric Fluoride * Hydrofluoric Acid * Lead Fluoborate
* Lead Fluoride * Sodium Bifluoride * Sodium
Fluoride * Zinc Fluoride * Zinc Silicofluoride *
Zirconium Potassium Fluoride.
Appendix 2 - Potential Hazardous Polluting
Substances:
Aluminum Fluoride * Antimony Pentafluoride * Benfluralin
* Chlorflurazole * Cobaltous Fluoride * Stannous Fluoride
|
US
Federal Register
••
Note: Due to length, the following is a partial
list. Click here
to see full list of FR entries.
|
| Published
date |
Docket
Identification Number |
Details |
| October 10, 2007 |
EPA-HQ-OPP-2007-0833 |
Sodium Fluoride Risk Assessment; Notice of Availability and Risk Reduction Options. This notice announces the availability of EPA's risk assessment, and related documents for the pesticide Sodium Fluoride and opens a public comment period on these documents. Comments on the following documents are due by December 10.
• Sodium Fluoride Preliminary Risk Assessment for the Reregistration Eligibility Decision (RED) Document . Sept. 30, 2007 (87 pages)
• Revised Occupational and Residential Exposure Chapter for the Sodium Fluoride Reregistration Eligibility Decision (RED). Oct. 1, 2007 (12 pages)
• Environmental Fate Science Chapter for the Sodium Fluoride Reregistration Eligibility Decision (RED) Document. Sept. 25, 2007 (8 pages)
• Sodium Fluoride Toxicology Chapter for Issuance of the Reregistration Eligibility Decision (RED) Document. Sept. 30, 2007 (78 pages)
• Product Chemistry Science Chapter For: Sodium Fluoride Reregistration Eligibility Decision (RED). Sept. 25, 2007 (2 pages)
• Revised Ecological Hazard and Environmental Risk Assessment Science Chapter for the Sodium Fluoride Reregistration Eligibility Decision (RED). Sept. 25, 2007 (13 pages)
• Sodium Fluoride – Incident Report Summary. Aug. 3, 2007 (11 pages) |
| Sept
21, 2005 |
OPP-2005-0069 |
Revocation
of Pesticide "Inert" Tolerance Exemption. FINAL
RULE.
Sodium fluoride is one of 34 exemptions that EPA is revoking
from the requirement of a tolerance that were identified in
the Federal Register of June 1, 2005 (see below). EPA says it
is revoking sodium fluoride because it is not contained in any
product as an "inert" ingredient. However, sodium
fluoride is used as an active ingredient in wood preservatives
and is used on "right of ways" - see FAN
comments submitted to EPA. |
| August
8, 2005 |
OPP-2005-0069 |
Proposed
rule; reopening of comment period.
This document reopens the public comment period established
in the Federal Register issued on June 1, 2005 (see below).
In that document, EPA sought comment on a proposed rule revoking
34 exemptions from the requirement of a tolerance that are
associated with 31 inert ingredients because, according to
Agency records, these substances are no longer contained in
active FIFRA pesticide product registrations. The following
are the comments submitted on this proposal. The comments
are relevant to the "inerts" issue, and not sodium
fluoride. FAN's comments are relevant to both.
| Date
of Letter |
Letter
from |
Details |
Docket
No. |
| July
18, 2005 |
Inerts
Steering Committee
A
joint effort of CropLife America and the Chemical Producers
and Distributors Assoc. |
Requests
a 30-day extenstion |
OPP-2005-0069-0003 |
| July
27, 2005 |
Monsanto |
Monsanto
states that their pesticide products "do not list
any of the specific chemicals." However, they submitted
5 interesting questions to EPA. |
OPP-2005-0069-0004 |
| July
27, 2005 |
Dow
AgroSciences |
In
their support for this proposal Dow submits questions
on inerts to EPA. |
OPP-2005-0069-0005 |
| July
27, 2005 |
Dow
AgroSciences |
This
letter from Dow contains Confidential Business Information
which has been deleted from this document. |
OPP-2005-0069-0006 |
| August
1, 2005 |
Fluoride
Action Network Pesticide Project |
FAN
supports the proposal and submits several questions to
EPA on the use of sodium fluoride. |
OPP-2005-0069-0007 |
|
| June
1, 2005 |
OPP-2005-0069 |
EPA
proposes to revoke sodium fluoride (40
CFR 180.920) as
an inert ingredient.
EPA is proposing to revoke 34 exemptions from the requirement
of a tolerance that are associated with 31 inert ingredients
because these substances are no longer contained in active
Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA)
pesticide product registrations. These ingredients are subject
to reassessment by August 2006 under section 408(q) of the
Federal Food, Drug, and Cosmetic Act (FFDCA), as amended by
the Food Quality Protection Act of 1996 (FQPA). Upon the issuance
of the final rule revoking the tolerance exemptions, the 34
tolerance exemptions will be counted as ``reassessed'' for
purposes of FFDCA's section 408(q). Comments
must be received on or before August 1, 2005.
EPA
is aware that inert ingredients are also contained in pesticide
adjuvant products which are not subject to registration under
FIFRA. The
Agency does not keep records of currently used adjuvants or
their ingredients, therefore, it has been unable to conclusively
confirm the use of adjuvants containing one of these inert
ingredients. Parties who know of currently used adjuvant products
that contain an inert ingredient subject to this proposal
are encouraged to submit documentation to EPA in the form
of the adjuvant product's current label and/or documentation
of the registration of the adjuvant product with a State adjuvant
registration program.
Also,
inert ingredient tolerance exemptions will be retained if
the tolerances or exemptions (which EPA refers to as ``import''
tolerances) are necessary to allow importation into the United
States of food containing such residues.
Through this proposed rule, the Agency is inviting individuals
who need these import tolerance exemptions to identify those
exemptions that are needed to cover imported commodities.
Parties
interested in the retention of any of the tolerance exemptions
subject to this notice should be aware that because these
ingredients are currently subject to reassessment under section
408(q) of FFDCA, additional data may be needed to support
retention of the exemption.
Reassessment activities for such ingredients must be completed
by August 2006. If the Agency is unable to determine that
the exemptions for these ingredients meet the FFDCA standard
for reassessment, the Agency will revoke the exemptions.
B.
When Do These Actions Become Effective?
EPA
is proposing that revocation of these tolerance exemptions
become effective on the day the final rule revoking these
tolerance exemptions is published in the Federal Register... |
| April
28, 2004 |
OPP-2003-0368 |
Pesticides;
Tolerance Exemptions for Active and Inert Ingredients for Use
in Antimicrobial Formulations (Food-Contact Surface Sanitizing
Solutions). FINAL RULE.
--
Stabilizer
carrier for formulations used
before crop emerges from soil.
Not
more than 0.25% of pesticide formulation. For
use in
pesticide formulations applied to growing crops only: |
| July
30, 2003 |
OPP-2002-0327 |
US
EPA's Pesticide Reregistration Performance Measures and Goals.
Candidate for Reregistration Eligibility Decision (RED) in Fiscal
Year 2004
which
runs
from October 1, 2003, through September 30, 2004. |
| April
16, 2003 |
OPP-2003-0116.
|
Requests to voluntarily
cancel certain pesticide registrations. EPA is issuing
a notice of receipt of request by registrants to voluntarily
cancel certain pesticide registrations. Unless a request is
withdrawn by October 13, 2003, or May 16, 2003 for EPA Registration
Numbers: 003008-00021, 075341-00001,
and 075341-00007, orders will be issued canceling these
registrations.
| Product |
Registration
Number |
Company
Name and Address |
| Hollow
Heart Concentrate |
075341-00001 |
Osmose
Utilities Services, Inc.
980 Ellicott Street,
Buffalo, NY 14209. |
| Osmoplastic
SD Wood Preserving Compound |
075341-00007
|
|
| Sept
5, 2001 |
OPP-301166 |
Proposed
Pesticide Temporary Tolerances for residues resulting from
the post harvest treatment with sulfuryl fluoride:
It is important to read the updated toxicological
risk assessment on inorganic fluoride prepared by EPA and
published in this issue of the FR. |
| Dec
21, 2000 |
TMD-00-02
(USDA) |
Approved
for use in US National Organic Standards. USDA National Organic
Program. - FINAL RULE.
The National Organic Program allows the use of all US EPA
"List 4 Inerts" in organic agriculture. Sodum fluoride
is a "List 4 Inert." Because "Inerts"
are considered confidential proprietary information, the public
is denied the right to know which crops they are used on.
The Final Rule states:
"In
this final rule, only EPA List 4 Inerts are allowed as ingredients
in formulated pesticide products used in organic crop and
livestock production. The allowance for EPA List 4 Inerts
only applies to pesticide formulations..." (see
last paragraph on page 248).
|
••
Note: Due to length, the above is a partial list.
Click here
to see full list of FR entries.
|
|