|
Return to Pyridalyl
Adverse Effects
ACTIVITY: Iinsecticide
(unclassified)
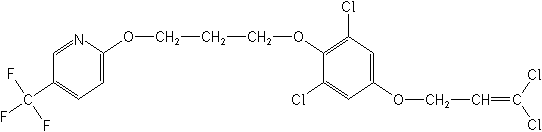
CAS Name: 2-[3-[2,6-dichloro-4-[(3,3-dichloro-2-propenyl)oxy]phenoxy]propoxy]-5-(trifluoromethyl)pyridine
Structure:
| Adverse
Effects:
Blood
Body Weight Decrease
Endocrine: Adrenal
Endocrine: Ovary
Endocrine: Testes
Kidney
Liver
Lung
Reproductive
Spleen
Environmental
- persistence in soil |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Pending |
| Japan:
Maximum Residue Levels (MRLS) |
Cabbage
• Chinese cabbage • Eggplant • Japanese radish
(including Radish) (leaf and root) • Lettuce (Cos and
Leaf) • Pimento (Sweet pepper) • Strawberry
•
Tomato •
Welsh (including Leek) |
| Other
Information |
| Molecular
Formula: |
C18H14Cl4
F3 NO3 |
| Entry
Year: |
2001
|
| Inventing
Company: |
Sumitomo
|
| Manufacturers: |
Valent |
| Other
Names: |
Pyridanil
S-1812 |
| Of
special interest: |
| PAN
Data |
| 2004:
Evaluation
Report. PYRIDALYL. January 14, 2004. Japan Food Safety Commission.
Pesticides Expert Committee. |
| 2004
- Abstract:
The discovery of pyridalyl: a novel insecticidal agent for controlling
lepidopterous pests by N Sakamoto et al. Pest Management Science,
January 2004, vol. 60, no. 1, pp. 25-34(10). |
| Dec
5, 2003 - Valent petitioned EPA for first
time tolerances for food commodities in the US. See Federal
Register below. |
| USDA-
funded field trials for new pesticide Pyridanil (now Pyridalyl)
from Sumitomo Chemical |
| August,
2001 - IR-4
New Products/Transitional Solution List.
This list contains brief descriptions
of numerous new pest control materials that have been introduced
over the last several years. Additionally, it contains information
on some "older" crop protection chemicals that are
believed to have room for new uses. This List includes
Pyridalyl
(S-1812) |
Metabolites
- abbreviations |
Chemical
name |
| S-1812-Py-OH |
2-[3-[2,6-dichloro-4-[(3,3,-dichloroallyloxy)]phenoxy]propoxy]
-5-(trifluoromethyl)-3-pyridynol |
| S-1812-DP
|
3,5-dichloro-4-
[3-(5-trifluoromethyl-2-pyridyloxy)]- propoxyphenol
[The unchanged form of the compound was
the main components in the feces, and a major metabolite]
[detected in the extracts of rat liver,
kidney, lung, whole blood, and fat] |
| S-1812-DP-Me |
2-[3-(2,6-dichloro-4-methoxyphenoxy)propoxy]-5-
(trifluoromethyl)pyridine |
| S-1812-Ph-CH2COO
H |
2-[3,5-dichloro-4-[3-(5-trifluoromethyl-2-pyridinoxy)]-propoxy]phe
noxy]acetic acid
[detected in the extracts of rat liver,
kidney, lung, whole blood, and fat] |
| HPHM |
3-[2,6-dichloro-4-(3,3-dichloroprop-2-enyloxy)phenoxy]-
propanol
[detected in the extracts of rat liver,
kidney, lung, whole blood, and fat] |
| DCHM |
3-[2,6-dichloro-4-(3,3-dichloroprop-2-propanil)oxy]phenol
|
| S-1812-PYP |
3-(5-trifluoromethyl-2-pyridyloxy)propanol
|
| TPPA |
3-(5-trifluoromethyl-2-pyridyloxy)propionic
acid |
| HTFP |
5-trifluoromethyl-2-hydroxypyridine
|
| HPDO |
5-trifluoromethyl-3-hydroxy-2-pyridone
|
| N-methyl-HTFP |
5-trifluoromethyl-N-methyl-2-pyridone |
| N-methyl-HPDO |
5-trifluoromethyl-3-hydroxy-N-methyl-2-pyridone
|
| Ref:
Evaluation
Report. PYRIDALYL. January 14, 2004. Japan Food Safety Commission.
Pesticides Expert Committee. |
| US
Federal Register |
| Date
Published |
Docket
Identification Number |
Details |
Dec
5, 2003
|
OPP-2003-0276
|
Valent
USA Corp.
Two Pesticide tolerance
petitions: PP 2F6459 and PP 2E6592
| Commodity |
PPM |
| Cottonseed |
0.4
|
| vegetable,
fruiting, group 8 |
1.1 |
vegetable,
leafy,
except Brassica, group 4 |
20.0
|
Brassica,
head and stem,
subgroup 5A |
5.0
|
| Brassica,
leafy greens, subgroup 5B |
30.0 |
| turnip
greens |
30 |
| meat
|
0.04
|
| meat
by-products |
0.05 |
| animal
fat |
1.0
|
| whole
milk |
0.1 |
and
to establish tolerances for residues of the insecticide
chemical pyridalyl plus the metabolite
S-1812-DP, 3,5-dichloro-4-[3-(5-trifluoromethyl-2-pyridyloxy)]propoxy
phenol, |
| cotton
gin by-products |
23.0 |
•
Genotoxicty... The
test systems assayed did not show any evidence of pyridalyl
genotoxicity except
the in vitro mammalian cytogenetics (chromosome aberration)
assay.
•Subchronic
toxicity.
-- Pyridalyl technical was tested in rats
in a 3-month feeding study. Effects included decreased
body weight gain, altered blood biochemistry, increased relative
liver weight and histopathological changes in the liver, ovary,
adrenal and lung. The NOAEL is 100 ppm
(5.56 mg/kg/day in males and 6.45 mg/kg/day in females).
-- A 13-week
feeding study in mice
was conducted. Effects included decreased body weight
gain, hematological and blood biochemical effects,increased
liver weight, decreased kidney and ovary weights and histopathological
changes in liver, kidney, ovary and adrenal. The
NOAEL is 70 ppm in males (8.169 mg/kg/day)
and 700 ppm in females (86.78 mg/kg/day).
--
A 13-week oral (capsule) toxicity study
was conducted in
dogs. Effects included decreased
body weight gain, clinical signs indicative of respiratory
distress, hematological and blood biochemistry effects, increased
liver, lung and kidney weights and histopathological alterations
of the lung, kidney, adrenal and liver. The
NOAEL was 10 mg/kg/day.
•Chronic toxicity.
-- In a 104-week combined chronic/oncogenicity
study in rats,
effects included decreased body weight
gain, increased frequency of rearing (high dose females only),
hematological alterations and histopathological alterations
of the spleen. No oncogenicity was found. The NOAEL
for this study is 100 ppm (3.4 mg/kg/day in males and 4.1
mg/kg/day in females).
-- Pyridalyl was administered in the
diet to mice
for 78-weeks. Effects
included decreased body weight
gain and food consumption/efficiency, and increased liver
and kidney weights. The NOAEL of the study was 50 ppm
(5.04 mg/kg/day in males and 4.78 mg/kg/day in females)
-- Pyridalyl was administered for 12-months
by capsule to
dogs. There were alterations
in blood biochemistry (alkaline
phosphatase and alanine aminotransaminase) and increased
liver weights. The NOAEL of the study was 20 mg/kg/day.
• Metabolite toxicology.
Two metabolites of pyridalyl, 2-hydroxy-5-trifluoromethylpyridine
(HTFP) and HPDO
that occur in extremely low levels in plants and animals,
were also tested for genetic toxicity. Both
metabolites were positive in the bacterial assay, but
were negative in the mammalian mutagenesis assay. One
metabolite, HPDO,
was positive in the chromosome aberration test.
• Endocrine
disruption. Data from the rat
reproduction and subchronic studies indicated that pyridalyl
may effect lipid metabolism and, consequently, hormone levels.
These in vivo results suggested that S-1812
may have a modulating activity on steroid biosynthesis
at doses generally exceeding MTD... studies
support the conclusion that S-1812 is not an endocrine disruptor
in in vivo mammalian systems. Although, very weak inhibition
of a single steroid biosynthesis pathway was observed in the
in vitro study, effects possibly related to this conversion
in mammalian systems were observed only at dose levels that
greatly exceeded the maximum tolerated dose. |
|