|
Return to Adverse
Effects
ACTIVITY:
Herbicide
(pyrazolylphenyl)
CAS Name
for
Pyraflufen:
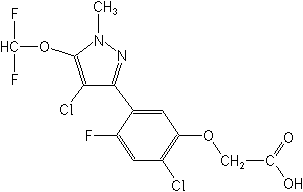
[2-chloro-5-[4-chloro-5-(difluoromethoxy)-1-methyl-1H-pyrazol-3-yl]-4-fluorophenoxy]acetic
acid
NOTES:
This compound is normally used as a salt or an ester, the identity
of which should be stated, for example pyraflufen-ethyl.
Structure
for Pyraflufen:
| Adverse
Effects:
Body
Weight Decrease
Blood
Cancer: Likely
to be Carcinogenic to Humans:
LIVER
Eye
Kidney
Liver
|
| Environmental
Effects:
The
acid metabolite has a potential for leaching which might require
particular attention in vulnerable areas to ensure protection
of groundwater. |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Yes |
| US
EPA PC Code: |
030090
|
| US
Tolerances: |
CFR
180.585 |
Registered
use in
(includes only a limited list of countries)
|
Japan,
US |
| Japan's
Maximum Residue Levels (MRLs) |
Apple,
Barley, Chestnut, Grape, Grapefruit, Japanese persimon, Lemon,
Lime, Mume plum, NATSUDAIDAI (whole), Orange (including Navel),
Other citrus fruits, Peach, Pear, Rice, UNSHU orange, Wheat |
US
Maximum Residue Levels permitted
in food commodities
|
13
residue tolerances for:
Corn, Cotton, Potato, Soybean |
| Other
Information |
| Entry
Year: |
1990 |
| Inventing
Company: |
Nihon
Nihyaku |
| Manufacturers: |
Nichino
America
Valent |
| Other
Names: |
Ecopart
Thunderbolt
ET-751
NH-9301
S-3153 Flufenpyr-ethyl Technical
S-3153 WDG Herbicide
S-3153 Atrazine WDG |
| Of
special interest: |
| PAN
Data |
| Material
Safety Data Sheets & Labels |
| May
17, 2005 -
New York state registration
of pyraflufen-ethyl contained in ET Herbicide Defoiliant (EPA
Reg. No. 71711-7). |
| Fact
Sheet from Washington State Department of Transportation.
This fact sheet is titled "Pyraflufen."
However the pesticide it refers to is Edict, which contains
pyraflufen-ethyl. |
| June
21, 2004 - Summary
of Toxicological Data, California EPA |
Cancer
Classification: ``Likely to be Carcinogenic to Humans'' by the
oral route.
Ref: Federal
Register: April 30, 2003. Pyraflufen-ethyl; Pesticide Tolerance.
Final Rule. |
| July
2, 2002 -
Review report for the active substance pyraflufen-ethyl.
European Commission Health & Consumer Protection Directorate-General. |
| October
2001 - Glossary
of Pesticide Chemicals - A listing
of pesticides subject to analysis of residues in foods and feeds
by the US Food and Drug Administration. |
| August
2001 - IR-4
New Products/Transitional Solution List -
This list contains brief descriptions of numerous new pest control
materials that have been introduced over the last several years.
Additionally, it contains information on some "older"
crop protection chemicals that are believed to have room for
new uses. This List includes:
Pyraflufen-ethyl
|
| See
also: Pyraflufen |
Pyraflufen-ethyl:
(ethyl
2-chloro-5-(4-chloro-5- difluoromethoxy-1-methylpyrazol-3-yl)-4-fluorophenoxyacetate)
US
EPA identified one metabolite: E-1,
(2-chloro-5-(4-chloro-5-difluoromethoxy-1- methylpyrazol-3-yl)-4-fluorophenoxyacetic
acid)
Pyraflufen-ethyl
belongs to the protox inhibitor class of compounds, and chemically
is a 3-phenylpyrazole. The herbicidal activity of protox inhibitors
is due to the inhibition of protoporphyrinogen IX oxidase.
Ref: US EPA, Federal Register: Nov
20, 2002
The
European
Commission identified 3 other metabolites and offered the
following comments:
E2: 2-chloro-5-(4-chloro-5-difluoromethoxy-1-methylpyrazole-3yl)-4-fluorophenol
E3: 4-chloro-3-(4-chloro-2-fluoro-5-methoxyphenyl)-5-difluoromethoxy-1-methylpyrazole
E9: 2-chloro-5-(4-chloro-5-difluoromethoxylpyrazole-3yl)-4-fluorophenoxyacetic
acid.
Pyraflufen-ethyl
was practically completely degraded into E1 metabolite. E1
metabolite was to a large extent degraded into metabolite
E2 and to a minor extent into metabolite E9. Metabolite E2
was almost completely degraded into metabolite E3. In the
aerobic soil metabolism studies, soil bound residues were
formed in percentages ranging from 15-17% and cumulative amounts
of CO2 ranged from 2 to 9%. The maximum levels and half-lives
of E1, E2 and E3 in laboratory studies with aerobic soils
are given in the following table.
| - |
max.
percentage (%) |
half-life
(days) at 20oC |
| E1 |
78 |
16
- 53 |
| E2 |
39 |
6
- 11 |
| E3 |
69 |
158
- 442 |
The half-lives
of E2 and E3 given in this table were measured in studies
in which either E2 or E3 was added to the soil. It was not
attempted to derive half-lives of E2 and E3 from metabolism
studies with the parent compound. However, the results of
studies with the parent compound are not consistent with the
half-lives of 6-11 days reported above for E2: in studies
with the parent compound the percentage E2 after 100 days
was 7, 12, 30 and 39% in four different soils. |
US
Federal Register
••
Note: Due to length, the following is a partial
list. Click here
to see full list of FR entries.
|
| Date
Published |
Docket
Identification Number |
Details |
| June 27, 2007 |
EPA-HQ-OPP-2007-0366 |
Nichino
America, Inc. Pesticide
Petition. PP 7F7190. Proposal to establish a tolerance
for residues of the herbicide, pyraflufen-ethyl and its acid
metabolite, E-1 (2-chloro-5-(4-chloro-5-difluoromethoxy-(1-methyl-1H-
pyrazol-3-yl)-4-fluorophenoxyacetic acid), expressed in terms
of the parent in or on food commodities:
| Soybeans, forage |
0.05 ppm |
| soybeans, hay |
0.1 ppm |
grass, forage, crop
group 17
|
1.0 ppm |
| grass, hay, crop
group 17 |
1.2 ppm |
Aqueous organic solvent extraction, column
clean up, and quantitation by GC is used to measure and evaluate
the chemical residues.
|
| May
12, 2004 |
OPP-2004-0094 |
Nichino
America. Pesticide
Tolerance. FINAL
RULE.
-- Pyraflufen-ethyl
has been classified as a ``likely
to be carcinogenic to humans'' by the oral route of
exposure. The
exposure from pyraflufen-ethyl residues in food results in
a cancer risk in the range of 1 in 1 million and is not a
concern.
-- Tolerances have been established (40 CFR 180.585) for the
combined residues of pyraflufen-ethyl and its acid metabolite,
E-1 (2-chloro-5-(4-
chloro-5-difluoromethoxy-1-methyl-1H- pyrazol-3-yl)-
4-fluorophenoxyacetic acid), expressed as the ester equivalent,
in or on a variety of raw agricultural commodities.
--
Pyraflufen-ethyl is currently registered for use on the following
residential non-dietary sites: Airports, nurseries, ornamental
turf, golf courses, roadsides, railroads, noncrop land, and
uncultivated agricultural areas.
-- EPA determined that the 10X SF to
protect infants and children should be removed and instead,
a different additional safety factor of 1X should be used.
The FQPA factor is removed because: There is no evidence of
increased susceptibility of rat or rabbit fetuses following
in utero exposure in the developmental studies with pyraflufen-ethyl;
there is no evidence of increased susceptibility of young
rats in the reproduction study with pyraflufen-ethyl; there
are no residual uncertainties identified in the exposure databases;
the dietary food exposure assessment is expected to be conservative,
tolerance-level residues and 100 PCT information were used;
and dietary drinking water exposure is based on conservative
modeling estimates.
| Commodity |
PPM
- Final Rule |
PPM
- Proposed |
| Wheat,
forage |
0.1 |
0.01 |
| Wheat,
grain |
0.01 |
0.01 |
| Wheat,
hay |
0.1 |
0.01 |
| Wheat,
straw |
0.01 |
0.01 |
|
| May
21, 2003 |
OPP-2003-0163 |
Nichino
America.
Pesticide
Tolerance. FINAL
RULE. This regulation establishes
a tolerance for combined residues of pyraflufen and pyraflufen-ethyl
in or on
-- Cotton, gin byproduct............ 1.5
-- Cotton, undelinted seed........0.04
-- Cancer Classification:
``Likely to be Carcinogenic to
Humans'' by the oral route Q1*
= 3.32 x 10-2 (mg/kg/day)-1
-- Inhalation1 long-term (< 6 months) Oral NOAEL= 20 1X
Mouse carcinogenicity. LOAEL = 98 mg/kg/day based
on liver toxicity.
-- Inhalation1 intermediate-term (1-6 months) Oral NOAEL=
20 1X Mouse carcinogenicity. LOAEL = 98 mg/kg/day based on
liver toxicity at interim sacrifice.
-- Inhalation1 short-term (1-30 days)
Oral NOAEL= 20 1X Developmental toxicity- rabbit. LOAEL =
60 mg/kg/day based on decreases in bwt
and food consumption, GI observations, and abortions.
-- Chronic dietary. Mouse carcinogenicity. Chronic RfD = 0.20
mg/ kg/day. LOAEL = 98 mg/kg/day. based on liver
toxicity.
-- Pyraflufen-ethyl is currently
registered for use on the following residential non-dietary
sites: Airports, nurseries, ornamental
turf, golf courses, roadsides, and railroads. |
| April
30, 2003 |
OPP-2003-0110. |
Nichino
America. Pesticide
tolerances. FINAL RULE established for combined
residues of the herbicide pyraflufen-ethyl (ethyl 2-chloro-5-(4-chloro-5-difluoromethoxy-1-
methyl-1H-pyrazol-3-yl)-4-fluorophenoxyacetate) and its
acid metabolite, E-1 (2-chloro-5-(4-chloro-5-difluoromethoxy-1-methyl-1H-
pyrazol-3-yl)-4-fluorophenoxyacetic acid)•,
expressed as the ester equivalent in or on
|
Commodity |
Parts
per million |
| Corn,
field, forage |
0.01 |
| Corn,
field, grain |
0.01 |
| Corn,
field, stover |
0.01 |
| Potato |
0.02 |
| Soybean,
forage |
0.01 |
| Soybean,
hay |
0.01 |
| Soybean,
seed |
0.01 |
-- Cancer
Classification: ``Likely
to be Carcinogenic to Humans'' by the oral route.
-- Mouse Carcinogenicity LOAEL = 98
mg/kg/day based on liver toxicty
-- Developmental toxicity- rabbit.
LOAEL = 60 mg/kg/day based on decreases
in bwt and food consumption,
GI observations, and abortions
•
--
Note from FAN; this metabolite is Pyraflufen. |
••
Note: Due to length, the above is a partial list.
Click here
to see full list of FR entries.
|
|

