US
EPA's "Children's Environmental Exposure Research Study"
(CHEERS) in Jacksonville, Duval County, Florida.
A
storm of controversy has engulfed this CHEERS study. The
plans to conduct this 2-year study were formally ended on
April
8, 2005. It was designed to monitor developmental changes
in babies, from birth to age 3, who are exposed to pesticides
and household chemicals in their homes. Chemicals include
PFOS and PFOA.
See FAN's
updates on the controvesy.
|
About
the Teflon chemical PFOA
PFOA is not only used to manufacture Teflon, but is also a
breakdown product of chemicals used to coat food packaging,
including fast food like McDonald's, and stain-resistant coatings
for couches, carpets, and clothing. PFOA is broadly toxic.
It does not break down in the environment, and is considered
to be persistent over geologic time scales. It nearly universally
pollutes human blood and has a half-life in the body of more
than four years.
Ref: Environmental
Working Group |
"The PFOS story is likely to emerge
as one of the apocryphal examples
of 20th century experimentation
with widespread chemical exposures:
prolific use and almost no testing for safety,
until unexpectedly and almost serendipitously, it is discovered
as a contaminant
virtually everywhere. And as is often the case in these stories,
the company producing
PFOS products possessed information hinting at its risks but chose
not to share their
data with regulators or the public for years..."
Ref: the web home for the authors of Our
Stolen Future
http://www.ourstolenfuture.org/NewScience/oncompounds/PFOS/2001-04pfosproblems.htm
"PFOS
accumulates to a high degree
in humans and animals. It has an estimated
half-life of 4 years in humans.
It thus appears
to combine
persistence, bioaccumulation, and
toxicity properties to an extraordinary degree."
Ref: May 16, 2000. Phaseout of PFOS. Charles
Auer, USEPA, Director,
Chemical Control Division Office of Pollution Prevention and Toxics
(OPPT)
http://www.chemicalindustryarchives.org/dirtysecrets/scotchgard/pdfs/226-0629.pdf#page=
"...
As
more studies pour in, PFCs
[perfluorinatred chemicals]
seem destined to supplant DDT, PCBs, dioxin
and other chemicals
as the most notorious, global chemical contaminants ever produced.
Government
scientists are especially concerned because unlike any other toxic
chemicals,
the most pervasive and toxic members of the PFC family never degrade
in the environment.
The U.S. EPA peremptorily forced one member of this family off
the market in 2000: PFOS,
the active ingredient used for decades in the original formulation
of 3M’s popular Scotchgard
stain and water repellent. Shortly thereafter, 3M also stopped
manufacture of a related
perfluorochemical, called PFOA, that is now under intense regulatory
pressure at EPA.
3M formerly sold PFOA to DuPont, which has used PFOA for half
a century in the manufacture
of Teflon. (DuPont now makes the chemical itself at a new facility
in North Carolina.)
Ref: PFCs:
a family of chemicals that contaminate the planet;
online report from
the Environmental Working Group - see below under Resources.
Research
into the adverse health and ecological effects
of perfluorinated chemicals is in its infancy.
The majority of animal studies were done by 3M, its major producer,
and by DuPont, now the major producer of PFOA. DuPont is facing
a Class Action lawsuit
in Ohio for the contamination of drinking water supplies with
the PFOA Ammonium Perfluorooctanoate (commonly known as C8). 3M
is also at risk of lawsuits.
The
following PFOS and PFOA chemicals, with some exceptions, were
used as pesticides or as EPA "Inerts" in pesticide products,
until they were phased out of use since the story broke in 2000.
The exceptions are that some are still permitted to be used. "Inerts"
are used in pesticide products and can account for more than 99%
of a pesticide product. US EPA only allows the name of the "active
ingredient" in a pesticide to be public information. EPA
maintains strict confidentiality on the names of the "inerts"
used in specific pesticides and the public is denied the right
to know what food crops they are used on, or other products, used
in homes, schools, parks, etc, that they are in.
EPA stated in the Dec 9, 2002 Federal Register, that sulfluramid
will be phased-out by the year 2016. One rationale EPA cited was
that sulfluramid is packaged in child-resistant
containers. However, in August 2001, New York State Attorney General
Eliot Spitzer, levied the largest pesticide penalty in NY State
history - $950,000 - against S.C. Johnson for illegally distributing
unsafe sulfluramid roach baits. According
to Spitzer.
"This
product was marketed for home use and was labeled as child resistant
when it was not."
"... According to an EPA assessment, if a child ingested
the bait, he or she could suffer irreversible reproductive damage,
and boys could be rendered infertile."
Ref: August 22, 2001 Press Release. Office
of the New York State
Attorney General Eliot Spitzer.
http://www.fluorideaction.org/pesticides/sulfluramid.largest.fine.01.htm
Activity
CAS No. |
Name
of Inert used in pesticides |
Comments |
INERT
LIST 3
2991-51-7 |
Potassium
N-ethyl-N-
[(heptadecafluorooctyl)sulfonyl] glycinate
EPA
calls it:
Glycine, N-ethyl-N- [(heptadecafluorooctyl)sulfonyl]-, potassium
salt |
See
December
9, 2002, Federal Register: FINAL
RULE.
Perfluoroalkyl Sulfonates; Significant New Use Rule. |
INERT
LIST 3
1652-63-7
|
3-(((Heptadecafluorooctyl)
sulfonyl)amino)-N,N,N- trimethyl-1-
propanaminium iodide
EPA
calls it:
1-Propanaminium, 3- [[(heptadecafluorooctyl)sulfonyl]am
ino]-N,N,N-trimethyl-, iodide
|
See
December
9, 2002, Federal Register: FINAL
RULE.
Perfluoroalkyl Sulfonates; Significant New Use Rule.
US
EPA lists this as an active inert
in March 2005 |
| PESTICIDE
29457-72-5
|
Lithium
perfluorooctane sulfonate
EPA
calls it:
1-Octanesulfonic acid, 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-
heptadecafluoro-, lithium salt |
See
December
9, 2002, Federal Register: FINAL
RULE.
Perfluoroalkyl Sulfonates; Significant New Use Rule. |
| Insecticide
No
CAS No.
PAN
Data |
Perfluorooctane
sulfonate |
Insecticide
Breakdown product |
INERT
LIST 3
No
CAS No. |
2-[Methyl
(polyfluoroalkylsulfonyl )
amino]ethyl methacrylate, polym. w. heptyl
methacrylate, butyl methacrylate and N-
methylolacrylamide |
These
are the names that US EPA used to describe these "inerts".
When
it comes to "inerts" EPA generally uses the least
common name for a chemical.
In the Federal Registers that pertain to PFAS, EPA used
the chemical names as listed in the "Ninth
Collective Index."
These
PFAS chemicals cannot be identified in EPA lists published
in the Federal Register. |
EPA
Inert
used as a
Water Repellant
Agent
No
CAS No. |
2-[Methyl
[(perfluoroalkyl)alkyl(C2-
C8)sulfonyl] amino]alkyl(C2-C8)
acrylate„alkyl(C2-C8) methacrylates- N-
methylolacrylamide copolymer |
EPA
INERT
used as a
Defoaming Agent
No
CAS No. |
Mono-
and bis-(1 H, 1 H,2 H, 2 H-perfluoroalkyl)
phosphates where the alkyl group is even
numbered and in the C6-C12 range |
Acaricide
Insecticide
4151-50-2
|
Sulfluramid
1-Octanesulfonamide,
N-ethyl- 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8- heptadecafluoro- |
See
December
9, 2002, Federal Register: FINAL
RULE.
Perfluoroalkyl Sulfonates; Significant New Use Rule.
In this
Final Rule, EPA states: "All
pesticide products containing sulfluramid are under a specific
timeline to be phased out by 2016." |
Insecticide
Adjuvant
No
CAS No. |
Potassium
N-ethyl perfluoro octane sulfonamide acetate |
- |
| PFOS
•
Exposure to PFOS is ubiquitous, found in human and wildlife
tissues around the world.
•
PFOS is remarkably persistent. For example, PFOS does not
show the slightest sign of degradation when boiled in nitric
acid for an hour.
•
PFOS is known to be toxic in laboratory animals (rats, mice,
monkeys) at levels close to the range already found in animals
and people in the real world.
Ref:
Environmental Science and Technology, report by Rebecca
Renner
•
The
biological half-life (t1/2) of perfluorooctanoic acid (PFOA)
in male rats is 70 times longer than that in female rats.
Ref:
2002 Chem Biol Interact Mar 20;139(3):301-16. Sex
hormone-regulated renal transport of perfluorooctanoic acid;
by Kudo N, Katakura M, Sato Y, Kawashima Y.
US
EPA uses these terms:
PFAS to refer to any chain length, including higher
and lower homologues as well as C8
PFOS to represent only those chemicals which are
predominantly C8 - with
an eight-carbon chain length.
PFAS
include PFOSH, PFOSS,
POSF, higher and lower homologues
of PFOSH and POSF, and certain
other chemical substances, including polymers, that are
derived from PFOSH and its
homologues.
All
of the chemical substances listed in EPA's supplemental
proposed Significant New Use Rule (SNUR) have the potential
to degrade to PFOSH in the environment.
Information also suggests that these chemical substances
may be converted to PFOSH via incomplete oxidation during
the incineration of PFAS-containing materials.
Once PFOSH has been released to the environment,
it does not undergo further chemical (hydrolysis), microbial,
or photolytic degradation. PFOSH is highly persistent in
the environment and has a strong tendency to bioaccumulate...
Ref 1. Federal Register. March 11,
2002. Perfluoroalkyl
Sulfonates; Proposed Significant New Use Rule. Supplemental
proposed rule. |
PFOA
In June 2000, EPA indicated
that it was expanding its investigation of PFOS to encompass
other fluorochemicals, including PFOA, in order to determine
whether these other fluorochemicals might present concerns
similar to those found with PFOS.
...PFOA is used primarily to produce its
salts, which are used as essential processing aids in the
production of fluoropolymers and fluoroelastomers.
... PFOA is persistent in
the environment. It does not hydrolyze, photolyze, or biodegrade
under environmental conditions. Based
on recent human biomonitoring data provided by industry,
which found PFOA in the blood of workers and the general
population in all geographic regions of the United States,
exposure to PFOA is potentially nationwide, although the
routes of exposure for the general population are unknown.
... APFO
[the chemical of concern in the Class Action suit in Ohio]
is the most widely used salt of PFOA, and
most animal toxicity studies have been conducted with APFO.
An extensive array of animal toxicity studies have been
conducted in rodents and monkeys. These studies have shown
that APFO exposure can result in a variety of toxic effects
in animals including liver toxicity, developmental
toxicity, and immunotoxicity. In addition,
rodent bioassays have shown that chronic APFO exposure is
associated with a variety of tumor
types. The mechanisms of APFO tumorigenesis are not
clearly understood....
... The major fluoropolymers
manufactured using PFOA salts are polytetrafluoroethylene
(PTFE) and polyvinylidine fluoride
(PVDF). PTFE has hundreds of uses
in many industrial and consumer products, including soil,
stain, grease, and water resistant coatings on textiles
and carpet; uses in the automotive, mechanical, aerospace,
chemical, electrical, medical, and building/construction
industries; personal care products; and non-stick coatings
on cookware. PVDF
is used primarily in three major industrial sectors: Electrical/electronics,
building/construction, and chemical processing.
... PFOA can be commercially manufactured
by two major alternative processes: The Simons Electro-Chemical
Fluorination (ECF) process, and a telomerization process.
Releases from manufacturing processes are one source of
PFOA in the environment. Historically, most U.S. production
was by 3M using the ECF process. 3M discontinued its manufacture
of PFOA between 2000 and 2002, and other domestic producers
are using the telomerization process exclusively.
...EPA has also received
data which indicate that the 8-2 telomer alcohol... (CAS
No. 678-39-7)) although not itself made with PFOA, can be
metabolized by living organisms or biodegrade under environmental
conditions to produce PFOA (Refs. 8 and 9). Other
telomer chemicals have not been tested to determine whether
they may also metabolize or degrade to form PFOA. Telomers
are used widely in a range of commercial products, including
some that are directly released into the environment, such
as fire fighting foams, as well as soil, stain, and grease
resistant coatings on carpets, textiles, paper, and leather.
The extent to which these telomer-containing products
might degrade to release PFOA is unknown.
However, anecdotal evidence of the atmospheric presence
of telomer alcohols in a multi-city North American survey
suggests that telomers may be one source of environmental
PFOA
The members of the FMG
[Fluoropolymer Manufacturing Group] --Asahi Glass Fluoropolymers
USA, Inc.; Daikin America, Inc.; E.I. duPont de Nemours
& Company; and Dyneon LLC--indicated that they
and their parent companies represent most of the known use
of APFO for the production of fluoropolymers both in the
United States and worldwide.
The members of the TRP
[Telomer Research Program] --AGA Chemicals (Asahi Glass);
Clariant GmbH; Daikin America, Inc.; and E.I. duPont de
Nemours & Company--indicated
that they comprise the major telomer producers, and that
they are evaluating telomer products sold in the United
States to determine whether they contribute to significant
human or environmental exposure
to PFOA.
Ref: April
16, 2003. Federal Register. Perfluorooctanoic Acid (PFOA),
Fluorinated Telomers; Request for Comment, Solicitation
of Interested Parties for Enforceable Consent
Agreement Development, and Notice of Public Meeting. Docket
Number OPPT-2003-0012
|
The PFOA derivative of greatest concern and most wide spread
use is the ammonium salt (commonly known as C8, C-8, or APFO).
CAS No: 3825-26-1. The molecular structure is:
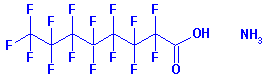
Resources:
•
Class
Action lawsuit against
Dupont and a local water company in Ohio, for the contamination
of drinking water supplies with the PFOA Ammonium perfluorooctanoate
(commonly known as C8). The C8 contamination originated from DuPont's
Washington Works facility in Wood County, West Virginia.
•
The Environmental Working Group (EWG)
produced an online expose of perfluorochemicals (PFCs) titled:
PFCs: a family of chemicals that
contaminate the planet. EWG highlighted
the PFOS/PFOA issue by associating it with the production of Teflon.
They state:
"Teflon itself is not PFOA (C8), but PFOA is used to manufacture
Teflon and is released to the air, along with other PFCs, when
Teflon cookware is heated to broiling temperatures. PFOA is
also emitted to air and water at Teflon manufacturing plants."
EWG's online report provides the most comprehensive
online accessibility to studies, data, and reports. The report
is available in the following sections:
After the announcement by 3M to phase-out PFOS
chemicals on May 16, 2000, the story is presented to the public
through several EPA Notices in the Federal Register. The first
was published on October 18, 2000, and provided
highly disturbing data on significant severe
adverse health effects from animal experiments. It also
provided EPA's list of the chemicals to be regulated. As this
drama unfolds, EPA extends the comment period on several occasions
to accomodate requests from various industries for. The requests
for exemptions, from companies in semiconductors,
imaging industries (such as Kodak), Boeing, Exxon, etc.,
are based on the lack of available alternatives. At the end of
this 2 year comment period, EPA agrees to the majority of these
exemptions, albeit
the levels should be greatly reduced compared to what was produced
in 2000. See a brief
review of the comments EPA received.
•
Abstracts
on PFOS and PFOA for the following years:
•
Timeline
for PFOS and PFOA chemicals
•
List
of Chemicals cited by EPA
•
Molecular
structures for some PFOS / PFOA chemicals
3M
produces sulfonated perfluorochemicals by an electrochemical
fluorination process. This process creates a complex and
variable mix of chemicals in which fluorine atoms replace
hydrogen atoms on the organic feedstock and carbon-carbon
bonds are rearranged. Because of the carbon-fluorine bond
formed by this process, the compounds created are considered
to be very stable. Perfluorochernicals have complete substitution
of fluorine for hydrogen... Fluorine's high electonegativity
confers a strong polarity to carbon-fluorine bonds, contributing
to the stability and nonreactive character of perfluorochemical
molecules. They are unusual as that perfluoroalkyl chains
are both oleophobic and hydrophobic. The addition of charged
moieties to the chain may affect the water solubility
of the shorter chains.
The highest volume sulfonated pduorochemical produced
by 3M is perfluorooctanesulfonyl fluoride (POSF). After
synthesis, it is used to create several product lines.
During their life cycles, POSF and POSF-based products
may degrade. If degradation occurs, current research suggests
peduorooctane sulfonate, (PFOS) and a few other perfluorinated
forms are degradation products... Degradation of sulfonated
perfluorochemicals is not complete but results in production
of other fluorochemicals. Studies suggest that compounds
made from POSF, a commercially important perfluorochemical
product and intermediate, are transformed during metabolism
to another sulfonated perfluomchemical, PFOS. PFOS does
not appear to further degrade except by incineration.•
...
The largest production of flurochemicals occurs at the
3M mmufacturing plant in Decatur, Alabama, and this plant
is the focus of current studies. During production, many
byproductc and waste products are formed. The volatile
waste products have been vented to the atmosphere in the
past but improvements are underway to capture and destroy
these releases by thermal oxidation. The tars are disposed
at hazardous waste landfills or treated by incinerattion.
The byproducts, many of which are incompletely fluorinated
with hydrogen atoms still present, are recycled back into
processes or partially degraded in stabilization processes
and discharged to wastewater treatment systems. The treatment
sludge is landfilled. Some of the non-POSF-based brproducts
are recovered and sold for secondary uses.
Note from FAN:
• Oleophobic =
[adj] lacking affinity for oils
•
Hydrophobic
= [adj] lacking affinity for water; tending to repel and
not absorb water; tending not to dissolve in or mix with
or be wetted by water
• At this time it is not known
if incineration "degrades" PFOS chemicals. From
what we know it is unlikely that it does.
| Table
5. Estimated 1998 Wastes Generated (in PFOS Equivalents)
at the Decatur [Alabama] Manufacturing Plant
(page 21) |
| Waste
type |
Estimated
PFOS Equivalents, lbs |
| Air
Emissions |
19,000
|
| Wastes
sent off-site to Incineration |
657,000
|
| Wastes
sent off-site to Landfills |
380,000
|
| Discharge
to River after Wastewater Treatment |
10,000
|
| Total
Wastes |
1,066,000
|
| Note:
The 10,000 lb/ryr of PFOS equivalents in the discharge
to the river are estimated releases to the environment
after wastewater treatment, not the lbs/yr generated
prior to treatment. |
Ref:
Sulfornated Perfluorochemicals
in the Environment: Sources, Dispersion, Fate and Effects.
Prepared by 3M. March 1,2000.
|
PFOS has been detected at low levels in the blood of humans and
wildlife throughout the United States, providing clear evidence
of widespread exposure to the chemical. PFOS
has been in commercial use since the 1950's, predominantly in
soil and stain-resistant coating products on fabrics, carpets,
and leather, and in grease and oil resistant coatings on paper
products, including food contact papers. Other uses leading
to environmental releases include fire fighting
foams. The various surface treatment uses constitute the
largest volume of PFOS production and are believed to present
the greatest potential for widespread human and environmental
exposure to PFOS. Studies are underway to determine the routes
of exposure which have led to the detection of PFOS in human and
animal blood. There are several potential
pathways that may account for the widespread exposure to PFOS
including: Dietary intake from the
consumption of food wrapped in paper containing PFOS derivatives;
inhalation from aerosol applications of PFOS-containing consumer
products; and inhalation, dietary, or dermal exposures resulting
from manufacturing, as well as industrial, commercial, and consumer
use and disposal of PFOS-derived chemicals and products.
Ref: October
18, 2000. Federal Register. Perfluorooctyl
Sulfonates. Proposed Significant New Use Rule (SNUR).
May 16, 2000 - 3M today
announced it is phasing out... perfluorooctanyl (PFOS)
... These include many Scotchguard products, such as soil, oil and
water repellent products; coatings used for oil and grease resistance
on paper packaging; fire-fighting foams; and specialty components
for other products. 3M said it plans to substantially phase out
production by the end of the year... "Our decision anticipates
increasing attention to the appropriate use and management of persistent
materials," said Dr. Charles Reich, executive vice president,
Specialty Material Markets.... Sophisticated testing capabilities
- some developed in only the last few years -- show that this persistent
compound, like other materials in the environment, can be detected
broadly at extremely low levels in the environment and in people.
All existing scientific knowledge indicastes that the presence of
these materials at these very low levels does not pose a human health
or environmental risk...
Ref: 3M
phasing out some of its specialy materials.
3M press release,
Two 3M plants, located in Decatur, Alabama
and Antwerp, Belgium, have produced POSF from electrochemical
cell fluorination and then subsequently used POSF to produce various
products through polymerization processes. These POSF-based products
degrade or metabolize to PFOS. A third manufacturing plant, located
in Cottage Grove, Minnesota, has not
produced POSF, but has manufactured some POSF-based products...
Ref: Nov 21, 2002: Hazard
Assessment of Perfluorooctane Sulfonate (PFOS) and its Salts.
Organisation for Economic Co-operation and
Development (OECD). 362 pages. ENV/JM/RD(2002)17/FINAL
What is the mechanicism(s) of action
of perfluorinated chemicals that make them so biologically active
and potent? This is what we need to know and understand.
EWG states:
"So far, five different pathways have been identified that
might explain how PFOA causes cancer and other types of toxicity.
These include
• mitochondrial toxicity;
• cell membrane disruption that results in decreased cell
communication;
• peroxisome proliferation;
• increased levels of estrogen and decreased levels of
testosterone; and
• decreased thyroid hormone levels."
For the record, a 1983 abstract:
"The acute toxicities of single ip
injections of perfluorooctanoic (PFOA) and perfluorodecanoic (NDFDA)
acids were evaluated in male Fischer rats... NDFDA has unusually
high toxic potency for a perfluorinated hydrocarbon, and some
of the toxic effects caused by this acid are remarkably similar
to those seen with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).
The acute toxicity of NDFDA may be due to an ability to interfere
with fatty acid metabolism, and studies of its toxicity may be
valuable in helping to understand mechanisms of action of TCDD."
Ref: 1983. Toxicol Appl Pharmacol
Sep 30;70(3):362-72. The
acute toxicity of perfluorooctanoic and perfluorodecanoic acids
in male rats and effects on tissue fatty acids; Olson CT,
Andersen ME.
|

