| Abstracts
on PFOS and PFOA for the following years: |
|
|
2005 |
|
|
|
|
|
Note:
PFOS and PFOA are a class of perfluorinated chemicals that are
best known for their use in the production of Teflon and other
stain resistent materials. The interest of the FAN Pesticide
Project in this issue is due to the use of several of the PFOS
and PFOA chemicals as "inerts" in pesticides. However,
most, but not all, have been deleted from use since 2001. The
so-called "inerts" used in pesticides can account
for as much as 99%, or more, of a pesticidal formulation. US
EPA's policy is to allow the public information only on the
"active substance" and to deny the public the names
of the chemicals used as "inerts" in specific pesticide
products -- even though the majority of inerts are toxic and
biologically active.
•
See the molecular
structure for some of these chemicals
•
The following is a selected list of abstracts. For more see
PubMed
or Toxnet.
Environmental
Pollution
Article in Press, Corrected Proof. Available online
21 November 2005.
Perfluorooctanesulfonate
and related fluorochemicals in biological samples from
the north coast of Colombia
Jesus
Olivero-Verbel (a), Lin Tao (b), Boris Johnson-Restrepo(a,
b), Jorge Guette-Fernández (a), Rosa Baldiris-Avila
(a), Indira O'byrne-Hoyos (a) and Kurunthachalam Kannan
(b)
(a)
Environmental and Computational Chemistry Group, Department
of Chemistry, University of Cartagena, A.A. 6541 Cartagena,
Colombia
(b) Wadsworth Center, New York State Department of Health
and Department of Environmental Health Sciences, State
University of New York at Albany, Empire State Plaza,
PO Box 509, Albany, NY 12201-0509, USA
Perfluorinated
compounds are widespread pollutants of toxicological importance
that have been detected in environmental matrices. However,
little is known on their distribution in South America.
In this study, distribution of perfluorooctanesulfonate
(PFOS), perfluorooctanoic acid (PFOA), perfluorohexanesulfonate
(PFHxS), and perfluorooctanesulfonamide (PFOSA) was determined
in the bile of mullet, Mugil incilis, and in tissues of
pelicans (Pelecanus occidentalis) collected from North
Colombia. Analysis was performed by HPLC mass spectrometry
after ion-pair extraction. PFOS was found in all bile
samples and PFOA and PFHxS were detected at lower frequency.
Average concentrations of PFOS, PFOA, and PFHxS in bile
of fish from Cartagena Bay, an industrialized site, and
Totumo marsh, a reference site, were 3673, 370, 489 and
713, 47.4, 1.27 ng/mL, respectively. PFOS concentrations
in pelican organs decreased in the order of spleen > liver > lung > kidney > brain > heart > muscle.
These results suggest, for the first
time, that perfluorinated compounds are also found in
wildlife from Latin American countries. |
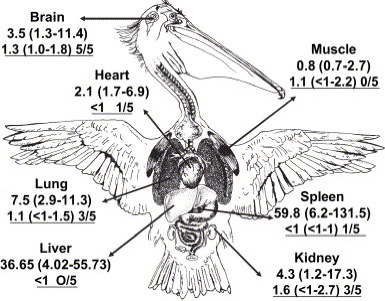 |
Fig.
5. Average PFOS and PFOSA concentrations (ng/g, wet weight)
in different tissues of pelicans from Cartagena Bay, Colombia.
PFOSA values and the number of positive samples detected
are underlined. |
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16221955&query_hl=7
Toxicol
Sci. 2005 Oct 12; [Epub
ahead of print]
Gene Expression Profiles in Rat Liver Treated
With Perfluorooctanoic Acid (PFOA).
Guruge
KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PK, Giesy
JP, Jones PD, Yamashita N.
Toxico-Biochemistry
Section, National Institute of Animal Health, Kannondai
3-1-5, Tsukuba, Ibaraki 305-0856, Japan.
Perfluorooctanoic
acid (PFOA; Pentadecafluorooctanoic acid ) is widely
used in various industrial applications. It is persistent
in the environment and does not appear to undergo further
degradation or transformation. PFOA is found in tissues
including blood of wildlife and humans, however, the
environmental fate and biological effects of PFOA remain
unclear. Microarray techniques of gene expression have
become a powerful approach for exploring the biological
effects of chemicals. Here, the Affymetrix, Inc. rat
genome 230 2.0 GeneChip was used to identify alterations
in gene regulation in Sprague-Dawley rats treated with
five different concentrations of PFOA. Male rats were
exposed by daily gavage to 1, 3, 5, 10, or 15 mg PFOA/kg,
bw/d for 21 d and at the end of the exposure, liver
was isolated and total liver RNA were used for the gene
chip analysis. Over 500 genes,
whose expression was significantly (P<0.0025) altered
by PFOA at two-fold changes compared to control, were
examined. The effects were
dose-dependent with exposure to 10mg PFOA/kg, bw/d,
causing alteration in expression of the greatest number
of genes (over 800). Approximately 106 genes and 38
genes were consistently up- or down-regulated, respectively
in all treatment groups. The largest categories of induced
genes were those involved in transport and metabolism
of lipids, particularly fatty acids. Other induced genes
were involved in cell communication, adhesion, growth,
apoptosis, hormone regulatory pathways, proteolysis
and peptidolysis and signal transduction. The genes
expression of which was suppressed were related to transport
of lipids, inflammation and immunity, and especially
cell adhesion. Several other genes involved in apoptosis;
regulation of hormones; metabolism; and G-protein coupled
receptor protein signaling pathways were significantly
suppressed.
PMID:
16221955 [PubMed - as supplied by publisher]
|
Mutation
Research/Genetic Toxicology and Environmental Mutagenesis
Volume 587, Issues 1-2 , 10 November 2005,
Pages 38-44
Genotoxic risk and oxidative DNA damage in HepG2
cells exposed to perfluorooctanoic acid.
Yao
X, Zhong L.
Department
of Toxicology, Dalian Medical University, 465 Zhongshan
Road, Dalian, 116027 Liaoning, China.
Perfluorooctanoic
acid (C(8)HF(15)O(2), PFOA) is widely used in various
industrial fields for decades and it is environmentally
bioaccumulative. PFOA is known as a potent hepatocarcinogen
in rodents. But it is not yet clear whether it is also
carcinogenic in humans, and the genotoxic effects of
PFOA on human cells have not yet been examined. In this
study, the genotoxic potential of PFOA was investigated
in human hepatoma HepG2 cells in culture using single
cell gel electrophoresis (SCGE) assay and micronucleus
(MN) assay. In order to clarify the underlying mechanism(s)
we measured the intracellular generation of reactive
oxygen species (ROS) using dichlorofluorescein diacetate
as a fluorochrome. The level of oxidative DNA damage
was evaluated by immunocytochemical analysis of 8-hydroxydeoxyguanosine
(8-OHdG) in PFOA-treated HepG2 cells. PFOA at 50-400muM
caused DNA strand breaks and at 100-400muM MN in HepG2
cells both in a dose-dependent manner. Significantly
increased levels of ROS and 8-OHdG were observed in
these cells. We conclude that
PFOA exerts genotoxic effects on HepG2 cells, probably
through oxidative DNA damage induced by intracellular
ROS.
|
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16040121&query_hl=10
Ecotoxicol
Environ Saf. 2005 Jul 22;
[Epub ahead of print]
The bioconcentration factor
of perfluorooctane sulfonate is
significantly larger than that of perfluorooctanoate
in wild turtles (Trachemys scripta elegans and
Chinemys reevesii): An Ai river ecological study in
Japan.
Morikawa
A, Kamei N, Harada K, Inoue K, Yoshinaga T, Saito N,
Koizumi A.
Department
of Health and Environmental Sciences, Kyoto University
Graduate School of Medicine, Yoshida Konoe, Sakyo, Kyoto
606-8501, Japan.
Turtles
rank high in the river food chain, and are suitable
for predicting the bioconcentrations of chemicals through
the food chain. Trachemys scripta elegans (N=46) and
Chinemys reevesii (N=51) were captured in a river in
Japan, from September to October 2003 and April to June
2004. Surface water samples were collected simultaneously
from the same sites at which the turtles were caught.
Serum perfluorooctane sulfonate (PFOS) ranged from 2.4
to 486mug/L, while water PFOS levels ranged from 2.9
to 37ng/L. The geometric mean (GM) (geometric
standard deviation, GSD) of the bioconcentration
factor (BCF) of PFOS was 10,964 (2.5). In contrast,
the perfluorooctanoate (PFOA) level in water ranged
from 16.7-87,100ng/L, and serum PFOA ranged from <0.2
to 870mug/L. The GM (GSD) of the BCF of PFOA was 3.2
(7.9). Furthermore, the BCF of PFOA decreased as the
PFOA level in the surface water increased. PFOS
could be preferentially bioconcentrated in biota, and
PFOA, slightly bioconcentrated.
PMID:
16040121 [PubMed - as supplied by publisher]
|
From
Science Direct
Regulatory
Toxicology and Pharmacology - 2005
Article in Press, Corrected Proof.
Available online 20 January 2005.
Toxicological profile of hydrofluoropolyethers
G.
Malinverno (a), I. Colombo (b), and M. Visca (b)
(a)
Solvay S.A., European Public
Affairs Brussels, Belgium
(b) Solvay Solexis R&D Centre,
Regulatory Affairs and Industrial Toxicology, Bollate,
Italy
Hydrofluoropolyethers (HFPE) are a family of linear
oligomeric fluorinated fluids comprising a chain of
difluoromethoxy and tetrafluoroethoxy repeating units
with terminal OCF2H end groups, each of which contains
an isolated hydrogen atom. These
fluids have been designed as low environmental impact
substitutes for perfluorinated organic substances in
a number of applications including heat transfer and
fire suppression agents, and as a solvent. The
toxicological profile of these new fluids has been evaluated
and is presented in this paper. Acute toxicity tests
have been performed on Sprague–Dawley Crl: CD
(SD) BR rats using oral, dermal, and inhalation routes.
No deaths were recorded even at the highest tested concentrations,
and the resultant LD50/LC50 values were >5000 mg/kg
(oral), >2000 mg/kg (dermal), and >26,411 ppm
(inhalation: reversible anaesthetic effects, e.g., lethargy,
seen at this exposure concentration). Other short-term
tests (skin and eye irritation, skin sensitisation,
genotoxicity tests in vitro and in vivo, cardiac sensitisation)
were also performed, and no hazardous properties were
identified. Effects of repeated exposure by inhalation
were examined in rats over test periods of 5, 14, 28,
and 90 days. Effects on embryo–foetal development
in the rat have also been studied. The 28-day, 90-day
and developmental studies were performed using nominal
HFPE concentrations of 1000, 3300, and 10,000 ppm
(6 h/day: actual exposures confirmed by test atmosphere
analysis), and the highest tested concentration proved
to be an NOAEL in each study. Major
observed effects were elevated urinary (inorganic) fluoride
levels and increased liver weights with centrilobular
hepatocyte hypertrophy (considered an adaptive
response, linked to hepatic metabolism of absorbed material).
|
Full
report available at Science
Direct
Toxicology
. V 215, Issues 1-2 , 5 November 2005, Pages 149-169
Neonatal mortality from in utero exposure to
perfluorooctanesulfonate (PFOS) in Sprague–Dawley
rats: Dose–response, and biochemical and pharamacokinetic
parameters
Deanna
J. Luebker (a), Raymond G. York (b), Kristen J. Hansenc(c)
, John A. Moore (d), and John L. Butenhoff (a)
(a)
3M Medical Department,
Corporate Toxicology and Regulatory Services, 3M Center
Building 220-06-E-03, St. Paul, MN 55144, USA
(b) Argus Divison, Charles River
Laboratories, 905 Sheehy Drive Bldg A, Horsham,
PA 19044, USA
(c) 3M Drug Delivery Systems
Division, 3M Center Building 260-04-N-12, St. Paul,
MN 55144, USA
dHollyhouse, Inc., P. O. Box 474, Wicomico Church, VA
22579, USA
Perfluorooctanesulfonate (PFOS) is a widely distributed,
environmentally persistent acid found at low levels
in human, wildlife, and environmental media samples.
Neonatal mortality has been observed following PFOS
exposure in a two-generation reproduction study in rats
and after dosing pregnant rats and mice during gestation.
Objectives of the current study were to better define
the dose–response curve for neonatal mortality
in rat pups born to PFOS-exposed dams and to investigate
biochemical and pharmacokinetic parameters potentially
related to the etiology of effects observed in neonatal
rat pups. In the current study, additional doses of
0.8, 1.0, 1.2, and 2.0 mg/kg/day were included
with original doses used in the two-generation study
of 0.4 and 1.6 mg/kg/day in order to obtain data
in the critical range of the dose–response curve.
Biochemical parameters investigated in dams and litters
included: (1) serum lipids, glucose, mevalonic acid,
and thyroid hormones; (2) milk cholesterol; and (3)
liver lipids. Pharmacokinetic parameters investigated
included the interrelationship of administered oral
dose of PFOS to maternal body burden of PFOS and the
transfer of maternal body burden to the fetus in utero
and pup during lactation, as these factors may affect
neonatal toxicity. Dosing of dams occurred for 6 weeks
prior to mating with untreated breeder males, through
confirmed mating, gestation, and day four of lactation.
Dose levels for the dose–response and etiological
investigation were 0.0, 0.4, 0.8, 1.0, 1.2, 1.6, and
2.0 mg/kg/day PFOS. Statistically
significant decreases in gestation length were observed
in the 0.8 mg/kg and higher dose groups. Decreases
in viability through lactation day 5 were observed in
the 0.8 mg/kg and higher dose groups, becoming
statistically significant in the 1.6 and 2.0 mg/kg
dose groups. Reduced neonatal survival did not
appear to be the result of reductions in lipids, glucose
utilization, or thyroid hormones. The
endpoints of gestation length and decreased viability
were positively correlated, suggesting that late-stage
fetal development may be affected in pups exposed to
PFOS in utero and may contribute to the observed mortality.
Benchmark dose (BMD) estimates for decreased
gestation length, birth weight, pup weight on lactation
day 5, pup weight gain through lactation day 5, and
viability resulted in values ranging from 0.27 to 0.89 mg/kg/day
for the lower 95% confidence limit of the BMD5 (BMDL5).
Results of analyses for PFOS in biological matrices
indicate a linear proportionality of mean serum PFOS
concentration to maternal administered dose prior to
mating and through the first two trimesters of gestation.
However, at 21 days of gestation, mean serum PFOS concentrations
were notably reduced from values measured earlier in
gestation. Urinary and fecal elimination was low as
expected from prior observations in adult rats. Significant
transfer of PFOS from dam to fetus in utero was confirmed,
and results suggest that dam and corresponding fetal
body burdens, as indicated by serum and liver PFOS levels,
correlate with neonatal survival.
|
Full
report available at Science
Direct
Toxicology.
V 215, Issues 1-2 , 5 November 2005, Pages 126-148
Two-generation reproduction and cross-foster
studies of perfluorooctanesulfonate (PFOS) in rats
Deanna
J. Luebker (a), Marvin T. Case (a), Raymond G. York
(b), John A. Moore (c), Kristen J. Hansen (d), and John
L. Butenhoff (a)
(a)
3M Medical Department,
Corporate Toxicology and Regulatory Services, 3M Center
Building 220-06-E-03, St. Paul, MN 55144, USA
(b) Argus Division, Charles River
Laboratories, 905 Sheehy Drive Bldg A, Horsham,
PA 19044, USA
(c) Hollyhouse, Inc., P.O. Box 474, Wicomico Church,
VA 22579, USA
(d) 3M Drug Delivery Systems
Division, 3M Center Building 260-04-N-12, St. Paul,
MN 55144, USA
Perfluorooctanesulfonate (PFOS) is a persistent acid
found widely distributed in wildlife and humans. To
understand the potential reproductive and developmental
effects of PFOS, a two-generation reproduction study
was conducted in rats. Male and female rats were dosed
via oral gavage at dose levels of 0, 0.1, 0.4, 1.6,
and 3.2 mg/(kg day) for 6 weeks prior to mating,
during mating, and, for females, through gestation and
lactation, across two generations. Due to substantial
F1 neonatal toxicity observed in the 1.6 and 3.2 mg/(kg day)
groups, continuation into the second generation was
limited to F1 pups from the 0, 0.1, and 0.4 mg/(kg day)
groups. No adverse effects were observed in F0 females
or their fetuses upon caesarean sectioning at gestation
day 10. Statistically significant
reductions in body-weight gain and feed consumption
were observed in F0 generation males and females at
dose levels of 0.4 mg/(kg day) and higher,
but not in F1 adults. PFOS did not affect reproductive
performance (mating, estrous cycling, and fertility);
however, reproductive outcome,
as demonstrated by decreased length of gestation, number
of implantation sites, and increased numbers of dams
with stillborn pups or with all pups dying on lactation
days 1–4, was affected at 3.2 mg/(kg day)
in F0 dams. These effects were not observed in
F1 dams at the highest dose tested, 0.4 mg/(kg day).
Neonatal toxicity in F1 pups, as demonstrated by reduced
survival and body-weight gain through the end of lactation,
occurred at a maternal dose of 1.6 mg/(kg day)
and higher while not at dose levels of 0.1 or 0.4 mg/(kg day)
or in F2 pups at the 0.1 or 0.4 mg/(kg day)
dose levels tested. In addition to these adverse effects,
slight yet statistically significant
developmental delays occurred at 0.4 (eye opening)
and 1.6 mg/(kg day) (eye opening,
air righting, surface righting, and pinna
unfolding) in F1 pups. Based
on these data, the NOAELs were as follows: reproductive
function: F0 ≥ 3.2 and F1 ≥ 0.4 mg/(kg day);
reproductive outcome: F0 = 1.6 and F1 ≥ 0.4 mg/(kg day);
overall parental effects: F0 = 0.1 and F1 ≥ 0.4 mg/(kg day);
offspring effects: F0 = 0.4 and F1 ≥ 0.4 mg/(kg day).
To distinguish between maternal and pup influences
contributing to the perinatal mortality observed in
the two-generation study, a follow-up cross-foster study
was performed. Results of this study indicated that
in utero exposure to PFOS causally contributed to post-natal
pup mortality, and that pre-natal
and post-natal exposure to PFOS was additive with respect
to the toxic effects observed in pups.
•
Pinna definition: The ear or,
to be more precise, the part of the ear that projects
like a little wing from the head. In Latin, pinna
means wing.
|
Abstracts
and Poster presentations
Fluoros 2005 Abstractbook
(133 pages):
An International Symposium on Fluorinated Alkyl Organics
in the Environment
August 18-20, 2005
Toronto, Canada
Topics:
Environmental Fate and Transport
Analytical Chemistry & Monitoring
Toxicology
Risk Assessment and Regulatory Policy
|
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16382930&query_hl=14&itool=pubmed_docsum
Environ
Sci Technol. 2005 Dec 1;39(23):9101-8.
Perfluorinated compounds in the plasma
of loggerhead and Kemp's ridley sea turtles from the southeastern
coast of the United States.
Keller
JM, Kannan K, Taniyasu S, Yamashita N, Day RD, Arendt MD, Segars
AL, Kucklick JR.
Hollings
Marine Laboratory, National Institute of Standards and Technology,
Charleston, South Carolina 29412, USA. jennifer.keller@noaa.gov
Perfluorinated
compounds (PFCs) have been measured in blood of humans and wildlife
and are considered globally distributed contaminants. We examined
12 PFCs in the plasma of 73 loggerhead sea turtles (Caretta
caretta) and 6 Kemp's ridley sea turtles (Lepidochelys kempii)
captured from inshore waters of Core Sound,
North Carolina (NC), and offshore waters of South Carolina,
Georgia, and Florida (SC-FL). Perfluorooctanesulfonate
(PFOS) and perfluorooctanoic acid (PFOA) were the dominant compounds,
with respective mean concentrations of 11.0 ng/mL and 3.20 ng/mL
for loggerhead turtles and 39.4 ng/mL and 3.57 ng/mL for Kemp's
ridley turtles. Mean PFOS concentrations
were 2- to 12-fold higher than typical mean sigmaPCB concentrations
(approximately 5 ng/g wet mass) measured previously in sea turtle
blood. More than 79% of the samples had detectable levels of
perfluorocarboxylates (PFCAs) with 8-12 carbons, whereas only
17% or less of samples had detectable levels of PFCAs with 6
or 7 carbons. No samples had detectable levels of PFCAs
with 4 or 5 carbons. In loggerhead turtles, sigmaPFC concentrations
were not influenced by sex (p > 0.05), but were higher
in turtles captured from inshore waters of NC than in turtles
from offshore waters of SC-FL (p = 0.009). A backward
stepwise multiple regression model showed that sigmaPFC concentrations
were (1) significantly higher in Kemp's
ridley turtles than loggerhead turtles (p < 0.0001), (2)
higher in larger turtles (p = 0.018; carapace length used as
a proxy for age), and (3) higher in turtles captured toward
the north (p = 0.006). These findings suggest that bioaccumulation
of PFCs in sea turtles is influenced by species, age, and habitat.
PMID:
16382930 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16323762&query_hl=14&itool=pubmed_docsum
Environ
Sci Technol. 2005 Nov 15;39(22):8678-83.
Ratio of perfluorochemical concentrations
as a tracer of atmospheric deposition to surface waters.
Simcik
MF, Dorweiler KJ.
Division
of Environmental Health Sciences, School of Public Health, University
of Minnesota, MMC 807, 420 Delaware Street SE, Minneapolis,
Minnesota 55455, USA. msimcik@umn.edu
A
major question regarding the global distribution of perfluorochemicals
(PFCs) is one of transport. It has been suggested that atmospheric
transport of volatile precursor compounds to remote areas and
subsequent degradation to the nonvolatile PFCs is responsible
for contamination of biota. This paper presents surface water
PFC concentrations aimed at identifying tracers of atmospheric
sources. Concentrations of PFCs including perfluorocarboxylates
from C6 to C10 and perfluorooctane sulfonate (PFOS) are presented
here from urban surface waters with presumably both atmospheric
and nonatmospheric sources of PFCs, remote waters with only
atmospheric sources of PFCs, and Lake Michigan. Perfluoroheptanoic
acid (PFHpA) and perfluorooctanoic acid (PFOA) were detected
in all surface water samples, and PFOS was detected in all but
two samples. PFOS concentrations ranged from nondetect to 1.2
ng/L and from 2.4 to 47 ng/L in remote and urban surface waters,
respectively. PFOA concentrations ranged from 0.14 to 0.66 ng/L
and from 0.45 to 19 ng/L in remote and urban surface waters,
respectively. The ratio of PFHpA to PFOA increased with increasing
distance from nonatmospheric sources suggesting that it can
be used as a tracer of atmospheric deposition of PFCs to surface
waters. The ratio ranged from 0.5 to 0.9 in urban areas and
from 6 to 16 in remote areas. Applying this tracer to measurements
from Lake Michigan indicates that the primary source of PFCs
to Lake Michigan is nonatmospheric, most likely inputs from
wastewater treatment effluent.
PMID:
16323762 [PubMed - in process]
Full
report available free at Science
Direct
Chemosphere,
Volume 61, Issue 7 , November 2005,
Pages 974-984
Thermal degradation of fluorotelomer
treated articles and related materials
Takahiro
Yamada (a), Philip H. Taylor (a), Robert C. Buck (b), Mary A.
Kaiser (c) and Robert J. Giraud (d)
(a)
Environmental Engineering Group, University of Dayton Research
Institute, 300 College Park, Dayton, OH 45469-0114, United States
(b) DuPont
Chemical Solutions Enterprise, 4417 Lancaster Pike, BMP23-2233,
Wilmington, DE 19805, United States
(c) DuPont
Corporate Center for Analytical Sciences, P.O. Box 80402,
Wilmington, DE 19880-0402, United States
(d) DuPont
Engineering Technology, 1007 Market Street, Wilmington,
DE 19898, United States
This study reports the first known studies to investigate the
thermal degradation of a polyester/cellulose fabric substrate
(“article”) treated with a fluorotelomer-based acrylic
polymer under laboratory conditions conservatively representing
typical combustion conditions of time, temperature, and excess
air level in a municipal incinerator, with an average temperature
of 1000 °C or greater over approximately 2 s residence
time. The results demonstrate that the polyester/cellulose fabric
treated with a fluorotelomer-based acrylic polymer is destroyed
and no detectable amount of perfluorooctanoic acid (PFOA) is
formed under typical municipal incineration conditions. Therefore,
textiles and paper treated with such a fluorotelomer-based acrylic
polymer disposed of in municipal waste and incinerated are expected
to be destroyed and not be a significant source of PFOA in the
environment.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16249997&query_hl=4
Birth
Defects Res B Dev Reprod Toxicol. 2005
Oct 25;74(5):405-416 [Epub ahead of print]
Effects of prenatal perfluorooctane sulfonate
(PFOS) exposure on lung maturation in the perinatal rat.
Grasty
RC, Bjork JA, Wallace KB, Lau CS, Rogers JM.
Reproductive
Toxicology Division, NHEERL, Office of Research and Development,
U.S. EPA, Research Triangle Park, North Carolina.
BACKGROUND:
Perfluorooctane sulfonate (PFOS), found widely in wildlife and
humans, is environmentally and metabolically stable. Environmental
PFOS may be from its use as a surfactant, hydrolysis of perfluorooctanesulfonyl
fluoride, and degradation of N-alkyl-perfluorooctanesulfonamide
compounds formerly used in numerous applications. Prenatal exposure
to PFOS in rodents causes neonatal mortality; treatment on gestation
days (GD) 19-20 is sufficient to induce neonatal death in rats.
Affected pups are born alive but present with labored breathing.
Their lungs are pale and often do not expand fully on perfusion.
METHODS: Pregnant Sprague-Dawley rats received 0, 25, or 50
mg/kg/day PFOS/K(+) orally on GD 19-20. Lungs from GD 21 fetuses
and neonates were prepared for histology and morphometry. Rescue
experiments included co-administration of dexamethasone or retinyl
palmitate with PFOS. Pulmonary surfactant was investigated with
mass spectrometry in GD 21 amniotic fluid and neonatal lungs.
Microarray analysis was carried out on PND 0 lungs.
RESULTS: Histologically, alveolar walls
were thicker in lungs of PFOS-exposed newborns compared to controls.
The ratio of solid tissue:small airway was increased,
suggesting immaturity. Rescue studies were ineffective. Phospholipid
concentrations and molecular speciation were unaffected by PFOS.
No changes in markers of alveolar differentiation were detected
by microarray analysis.
CONCLUSIONS: Morphometric changes in lungs of PFOS exposed neonates
were suggestive of immaturity, but the failure of rescue agents
and normal pulmonary surfactant profile indicate that the labored
respiration and mortality observed in PFOS-treated neonates
was not due to lung immaturity.
PMID:
16249997 [PubMed - as supplied by publisher]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16252056&query_hl=4
J
Environ Monit. 2005;7(11):1074-1078.
Epub 2005 Sep 19.
Occurrence of perfluorosulfonates and
other perfluorochemicals in dust from selected homes in the
city of Ottawa, Canada.
Kubwabo
C, Stewart B, Zhu J, Marro L.
Health
Canada, Healthy Environments and Consumer Safety Branch, Safe
Environments Programme, Tunney's Pasture, Building No 8, Ottawa
(Ontario) PL 0800C, CanadaK1A 0L2. Cariton_Kubwabo@hc-sc.gc.ca.
A
series of perfluorinated compounds (PFCs) including perfluorooctane
sulfonate (PFOS) and perfluorooctanoic acid (PFOA) have been
recently measured in a variety of environmental samples and
biological matrices. In order to better understand the human
exposure routes of these chemicals, levels of PFOS, PFOA, perfluorobutane
sulfonate (PFBS), perfluorohexane sulfonate (PFHS) and perfluorooctane
sulfonamide (PFOSA) in house dust samples were investigated.
The data revealed a correlation between
the concentrations of PFCs and the percentage of carpeting in
the house; older houses tended to have less carpeting, hence
lower levels of these perfluorinated compounds in their dust.
PMID:
16252056 [PubMed - as supplied by publisher]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16245813&query_hl=4
Environ
Sci Technol. 2005 Oct 1;39(19):7439-45.
Perfluorinated alkyl substances in plasma,
liver, brain, and eggs of glaucous gulls (Larus hyperboreus)
from the Norwegian arctic.
Verreault
J, Houde M, Gabrielsen GW, Berger U, Haukas M, Letcher RJ, Muir
DC.
Norwegian
Polar Institute, Tromso, NO-9296, Norway, jonathan@npolar.no
Recent
environmental surveys have ascertained the widespread occurrence
of perfluorinated alkyl substances (PFAS) in tissues of wildlife
from the Arctic. In the present study, we investigated the distribution
of a suite of PFAS in plasma, liver, brain, and egg samples
from adult glaucous gulls (Larus hyperboreus), an apex scavenger-predator
seabird breeding in the Norwegian Arctic. Perfluorooctane
sulfonate (PFOS) was the predominant PFAS in all samples and
was present at concentrations that are the highest reported
thus far in any arctic seabird species and populations.
Among the body compartment/ tissue samples analyzed, PFOS
was highest in plasma (48.1-349 ng/g wet weight (ww)), followed
by liver approximately equal to egg > brain. Perfluorocarboxylic
acids (PFCAs) with 8-15 carbon (C) atoms were found, with the
highest concentrations determined in plasma (sum PFCA: 41.8-262
ng/g ww), whereas 5C- and 6C-PFCAs were below the limits of
detection. Perfluorobutane sulfonate, perfluorooctane sulfonamide,
and four saturated (8:2 FTCA and 10:2 FTCA) and unsaturated
(8:2 FTUCA and 10:2 FTUCA) fluorotelomer carboxylic acids were
not detected in any samples. Perfluorohexane sulfonate was measured
at concentrations up to 2.71 ng/g ww. The
accumulation profiles of PFCAs were characterized by high proportions
of the long and odd-numbered carbon-chain-length compounds,
namely perfluoroundecanoic (11C) and perfluorotridecanoic acid
(13C), although their individual contribution differed
between the matrixes analyzed. Current PFAS concentrations suggest
a bioaccumulation potential in Norwegian arctic glaucous gulls
that needs to be assessed as part of a broad organohalogen contaminant
cocktail with potential for mediating biological processes in
this vulnerable top-predator marine species.
PMID:
16245813 [PubMed - in process]
Full
report available at Science
Direct
Toxicology . V 176, Issue 3 , 15 July 2002, Pages 175-185
Interactions of flurochemicals with rat liver fatty
acid-binding protein
Deanna J. Luebker (a), Kris J. Hansen
(b), Nathan M. Bass (c), John L. Butenhoff (a) and Andrew M.
Seacat (a)
(a) 3M Medical Department, Corporate
Toxicology, 3M Center Building 220-2E-02, Saint Paul, MN 55144,
USA
(b) 3M Environmental Technology
and Safety Services, Building 02-3E-09, Saint Paul, MN 55144,
USA
(c) School of Medicine, University of California, San Francisco,
CA 94143, USA
Liver-fatty acid binding protein (L-FABP) is an abundant intracellular
lipid-carrier protein. The hypothesis that perfluorooctanesulfonate
(PFOS), perfluorooctanoate (PFOA), and certain related perfluorooctanesulfonamide-based
fluorochemicals (PFOSAs) can interfere with the binding affinity
of L-FABP for fatty acids was tested. The relative effectiveness
of PFOA, PFOS, N-ethylperfluorooctanesulfonamide (N-EtFOSA),
N-ethylperfluorooctanesulfonamido ethanol (N-EtFOSE), and of
the strong peroxisome proliferator Wyeth-14 643 (WY) to
inhibit 11-(5-dimethylaminonapthalenesulphonyl)-undecanoic acid
(DAUDA) binding to-L-FABP was determined. The dissociation constant
(Kd) of the DAUDA-L-FABP complex was 0.47 nM. PFOS exhibited
the highest level of inhibition of DAUDA-L-FABP binding in the
competitive binding assays, followed by N-EtFOSA, WY, and, with
equal IC50s, N-EtFOSE and PFOA. The in
vitro data presented in this study support the hypothesis that
these fluorochemicals may interfere with the binding of fatty
acids or other endogenous ligands to L-FABP. Furthermore, this
work provides evidence to support the hypothesis that displacement
of endogenous ligands from L-FABP may contribute to toxicity
in rodents fed these fluorochemicals.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16245810&query_hl=4
Environ
Sci Technol. 2005 Oct 1;39(19):7416-22.
Temporal and spatial trends of perfluorinated
compounds in ringed seal (Phoca hispida) from Greenland.
Bossi
R, Riget FF, Dietz R.
National
Environmental Research Institute, Frederiksborgvej 399, 4000-Roskilde,
Denmark. rbo@dmu.dk
Perfluorinated
compounds (PFCs), such as perfluorooctane sulfonate (PFOS) and
related compounds, have been identified as global pollutants
and have shown their bioaccumulation into higher trophic levels
in the food chain. PFCs have been found in remote areas far
from sources, such as the Arctic. In this study spatial and
temporal trends in the concentrations of selected PFCs were
measured using archived liver samples of ringed seal (Phoca
hispida) from East and West Greenland. The samples were collected
in four different years at each location, between 1986 and 2003
in East Greenland and between 1982 and 2003 in West Greenland.
PFOS was the major contributor to the burden of PFCs in samples,
followed by perfluoroundecanoic acid (PFUnA). Perfluorononanoic
acid (PFNA) and perfluorodecanoic acid (PFDA) were also detected
in most samples. Perfluorohexane sulfonate (PFHxS) and perfluorooctane
sulfonamide (PFOSA) were only found sporadically. Perfluorooctanoic
acid was not found in detectable concentrations in any sample.
Regression analysis of logarithmic transformed PFOS, PFDA, and
PFUnA median concentrations indicated a
significant temporal trend with increasing concentrations at
both locations. A spatial trend in PFOS concentrations
(ANOVA, p < 0.0001) was observed between the two sampling
locations, with significantly higher concentrations in seals
from East Greenland.
PMID:
16245810 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16227186&query_hl=7
Food
Addit Contam. 2005 Oct;22(10):1023-31.
Perfluorochemicals: Potential sources
of and migration from food packaging.
Begley
TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA.
US
Food and Drug Administration,
Center for Food Safety and Applied Nutrition, College Park,
MD 20740, USA.
Perfluorochemicals
are widely used in the manufacturing and processing of a vast
array of consumer goods, including electrical wiring, clothing,
household and automotive products. Furthermore, relatively small
quantities of perfluorochemicals are also used in the manufacturing
of food-contact substances that represent potential sources
of oral exposure to these chemicals. The most recognizable products
to consumers are the uses of perfluorochemicals in non-stick
coatings (polytetrafluoroethylene (PTFE)) for cookware and also
their use in paper coatings for oil and moisture resistance.
Recent epidemiology studies have demonstrated the presence of
two particular perfluorochemicals, perfluorooctane sulfonate
(PFOS) and perfluorooctanoic acid (PFOA) in human serum at very
low part per billion levels. These perfluorochemicals are biopersistent
and are the subject of numerous studies investigating the many
possible sources of human exposure. Among the various uses of
these two chemicals, PFOS is a residual
impurity in some paper coatings used for food contact and PFOA
is a processing aid in the manufacture of PTFE used for many
purposes including non-stick cookware. Little information
is available on the types of perfluorochemicals that have the
potential to migrate from perfluoro coatings into food. One
obstacle to studying migration is the difficulty in measuring
perfluorochemicals by routine conventional analytical techniques
such as GC/MS or LC-UV. Many perfluorochemicals used in food-contact
substances are not detectable by these conventional methods.
As liquid chromatography-mass spectrometry (LC/MS) develops
into a routine analytical technique, potential migrants from
perfluoro coatings can be more easily characterized. In this
paper, data will be presented on the types of perfluoro chemicals
that are used in food packaging and cookware. Additionally,
research will be presented on the migration or potential for
migration of these chemicals into foods or food simulating liquids.
Results from migration tests show mg kg(-1) amounts of perfluoro
paper additives/coatings transfer to food oil. Analysis of PTFE
cookware shows residual amounts of PFOA in the low microg kg(-1)
range. PFOA is present in microwave popcorn bag paper at amounts
as high as 300 microg kg(-1).
PMID:
16227186 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16213555&query_hl=7
Chemosphere. 2005 Oct 4; [Epub
ahead of print]
Perfluorinated chemicals in selected
residents of the American continent.
Calafat AM, Needham LL, Kuklenyik Z,
Reidy JA, Tully JS, Aguilar-Villalobos M, Naeher LP.
Division of Laboratory Sciences, National
Center for Environmental Health, Centers for Disease Control
and Prevention, 4770 Buford Hwy., NE, Mailstop F17, Atlanta,
GA 30341, USA.
Perfluorinated chemicals (PFCs) are used in multiple consumer
products. Perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic
acid (PFOA), the most widely studied PFCs, may be potential
developmental, reproductive, and systemic toxicants. Although
PFCs seem to be ubiquitous contaminants found both in humans
and animals, geographic differences may exist in human exposure
patterns to PFCs. We measured 11 PFCs in 23 pooled serum samples
collected in the United States from 1990 through 2002, and in
serum samples collected in 2003 from 44 residents from Trujillo,
Peru. PFOS and PFOA were detected in all the pooled samples;
perfluorohexane sulfonic acid (PFHxS) was detected in 21. Median
concentrations were 31.1 micrograms per liter (mug/l, PFOS),
11.6mug/l (PFOA), and 2mug/l (PFHxS). The 90th percentile concentrations
of PFCs in the 44 Peruvian residents were 0.7mug/l (PFOS), 0.1mug/l
(PFOA), and <0.3mug/l (PFHxS). The frequencies of detection
were 20% (PFOS), 25% (PFOA), and 9% (PFHxS). The
frequent detection of selected PFCs in the pooled samples from
the United States and the lack of clear concentration trends
based on a year of collection suggest a sustained widespread
exposure to these compounds among US residents, at least since
the 1990s. By contrast, the much
lower frequency of detection and concentration ranges of PFCs
in Peru suggest a lower exposure of Peruvians to PFCs compared
with North Americans. Genetic variability, diet, lifestyle,
or a combination of all these may contribute to the different
patterns of human exposure to PFCs in the United States and
Peru.
PMID: 16213555 [PubMed - as supplied by publisher]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16193761&query_hl=5
Environ
Toxicol Chem. 2005 Sep;24(9):2316-24.
Reproductive and developmental toxicity
and bioconcentration of perfluorooctanesulfonate in a partial
life-cycle test with the fathead minnow (Pimephales promelas).
Ankley
GT, Kuehl DW, Kahl MD, Jensen KM, Linnum A, Leino RL, Villeneuvet
DA.
Mid-Continent
Ecology Division, National Health and Environmental Effects
Research Laboratory, U.S. Environmental Protection Agency, 6201
Congdon Boulevard, Duluth, Minnesota 55804, USA. ankley.gerald@epa.gov
Perfluorooctanesulfonate
(PFOS) is a widespread environmental contaminant emanating from
the production and/or metabolism of fluorinated chemicals with
a variety of applications. The goal of this work was to assess
the toxicity and bioconcentration of PFOS in the fathead minnow
(Pimephales promelas). Sexually mature fish were exposed via
the water for 21 d to 0 (control), 0.03, 0.1, 0.3, or 1 mg PFOS/L,
and effects on reproductive capacity and endocrinology were
assessed. To determine possible developmental effects, a subset
of embryos from parental exposures at each test concentration
were held for an additional 24 d in the same PFOS treatments.
A concentration of I mg PFOS/L was lethal to adults within two
weeks. The 21-d 50% effect concentration (95% confidence interval)
for effects on fecundity of the fish was 0.23 (0.19-0.25) mg
PFOS/L. Exposure to PFOS caused various
histopathological alterations, most prominently in ovaries of
adult females. Adult males exposed to 0.3 mg PFOS/L for 21 d
exhibited decreased aromatase activity and elevated concentrations
of plasma 11-ketotestosterone and testosterone. No significant
adverse effects on survival or growth were observed in developing
fathead minnows held for 24 d at PFOS concentrations up to 0.3
mg/L. Adult fathead minnows readily accumulated PFOS from the
water. The largest concentrations of PFOS
were in blood, followed by liver and then gonad; for all tissues,
females accumulated higher concentrations than males.
Water and tissue concentrations of PFOS associated with effects
in this study exceeded those reported for samples collected
from the field by two to three orders of magnitude, suggesting
that the current risk of PFOS on aspects of fish reproduction
and development assessed in this study would be small.
PMID:
16193761 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16190216&query_hl=5
Environ
Sci Technol. 2005 Sep 1;39(17):6591-8.
Polyfluoroalkyl compounds in free-ranging
bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico
and the Atlantic Ocean.
Houde
M, Wells RS, Fair PA, Bossart GD, Hohn AA, Rowles TK, Sweeney
JC, Solomon KR, Muir DC.
Department
of Environmental Biology, University of Guelph, Guelph, Ontario
NIG 2W1, Canada.
Polyfluoroalkyl
compounds (PFAs) have been used for decades in industrial and
commercial products and are now detected worldwide. Concentrations
of two major PFA groups, carboxylic acids (PFCAs) and sulfonic
acids (PFSAs), were assessed in plasma of bottlenose dolphins
from the Gulf of Mexico (Sarasota Bay,
FL) and the Atlantic Ocean (Delaware
Bay, NJ, Charleston, SC, Indian River Lagoon (IRL), FL, and
Bermuda). Eight PFAs were detected
in the plasma of all dolphins. Perfluorooctane sulfonate (PFOS)
was the predominant compound at all locations (range from 49
ng/g wet weight (w.w.) in dolphins from Bermuda to 1171 ng/g
w.w. in plasma of animals from Charleston). Sum of PFA concentrations
were significantly higher in animals from Charleston compared
to IRL, Sarasota Bay, and Bermuda. Concentrations of
several PFAs were negatively associated with age in animals
from IRL and Charleston. No differences between gender were
observed for all compounds at all locations.
An increase in PFA concentrations was associated with a decrease
of blubber thickness in animals from Sarasota Bay and IRL.
Fluorotelomer 8:2 and 10:2 unsaturated
carboxylic acids (FTUCAs), known degradation products of fluorotelomer
alcohols and suspected precursors to PFCAs, were detected for
the first time at low concentrations in plasma of dolphins.
PMID:
16190216 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16170448&query_hl=5
Arch
Environ Contam Toxicol. 2005 Sep
16; [Epub ahead of print]
Alkaline Digestion and Solid Phase Extraction
Method for Perfluorinated Compounds in Mussels and Oysters from
South China and Japan.
So
MK, Taniyasu S, Lam PK, Zheng GJ, Giesy JP, Yamashita N.
Centre
for Coastal Pollution and Conservation, Department of Biology
and Chemistry, City University of Hong Kong, Tat Chee Avenue,
Kowloon, Hong Kong SAR, Peoples Republic of China, bhpksl@cityu.edu.hk.
Perfluorinated
compounds (PFCs), such as perfluorooctane sulfonate (PFOS),
have been identified in the coastal waters of China and Japan.
An alkaline digestion method, coupled with solid-phase extraction
(SPE), and high-performance liquid chromatography interfaced
with high-resolution electrospray tandem mass spectrometry was
developed to determine PFCs in mussel and oyster samples from
coastal waters of south China and Japan. These techniques produced
adequate recoveries and reporting limits with small quantities
of PFCs. Concentrations of individual
PFCs in mussels and oysters from south China and Japan ranged
from 113.6 to 586.0 pg/g, wet weight (ww) for PFOS, 63.1 to
511.6 pg/g, ww for perfluorohexane sulfonate, 9.3 to 30.1 pg/g,
ww for perfluorobutane sulfonate and 37.8 to 2957.0 pg/g, ww
for perfluorooctane sulfonamide. The quantification of
perfluorinated carboxylates was compromised by interferences
from carboxylates in the procedural blanks. Perfluoroundecanoate
and perfluorononanoate had relatively great blank interferences,
which resulted in relatively poor limits of quantification for
these compounds. Some PFCs were only identified in a limited
number of samples: perfluorododecanoate in samples from Tokyo
Bay, Japan (195.9 pg/g, ww); and perfluorodecanoate in Fuzhou,
China (131.7 pg/g, ww) and Tokyo Bay (118.6 pg/g, ww). The
greatest concentrations of perfluorooctanoate, perfluoroheptanoate,
and perfluorohexanoate were observed in samples from Tokyo Bay
and Bei Hai, south China.
PMID:
16170448 [PubMed - as supplied by publisher]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16201619&query_hl=5
Environ
Sci Technol. 2005 Sep 15;39(18):6978-84.
Tissue distribution of perfluorinated
chemicals in harbor seals (Phoca vitulina) from the Dutch Wadden
Sea.
Van
de Vijver KI, Hoff P, Das K, Brasseur S, Van Dongen W, Esmans
E, Reijnders P, Blust R, De Coen W.
Department
of Biology, University of Antwerp, Groenenborgerlaan 171, 2020
Antwerpen, Belgium. inneke.vandevijver@ua.ac.be
Perfluorinated
acids (PFAs) are today widely distributed in the environment,
even in remote arctic areas. Recently, perfluorooctane sulfonate
(PFOS) has been identified in marine mammals all over the world,
but information on the compound-specific tissue distribution
remains scarce. Furthermore, although longer perfluorinated
carboxylic acids (PFCAs) are used in industry and were shown
to cause severe toxic effects, still little is known on potential
sources or their widespread distribution. In this study, we
report for the first time on levels of longer chain PFCAs, together
with some short chain PFAs, perfluorobutane
sulfonate (PFBS) and perfluorobutanoate (PFBA), in liver,
kidney, blubber, muscle, and spleen tissues of harbor seals
(Phoca vitulina) from the Dutch Wadden Sea. PFOS was the predominant
compound in all seal samples measured (ranging from 89 to 2724
ng/g wet weight); however, large variations between tissues
were monitored. Although these are preliminary results, it is,
to our knowledge, the first time that
PFBS could be found at detectable concentrations (2.3 +/- 0.7
ng/g w wt) in environmental samples. PFBS was only detected
in spleen tissue. PFCA levels were much lower than PFOS concentrations.
The dominant PFCA in all tissues was PFNA (perfluorononanoic
acid), and concentrations generally decreased in tissues for
all other PFCA homologues with increasing chain length.
No clear relationship between PFOS levels in liver and kidney
was observed. Furthermore, hepatic PFDA
(perfluorodecanoic acid) levels increased with increasing body
length, but in kidney tissue, PFDA levels showed an inverse
relationship with increasing body length. These data
suggest large differences in tissue distribution and accumulation
patterns of perfluorinated compounds in marine organisms.
PMID:
16201619 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15973506&query_hl=4
Inflamm
Res. 2005 Jun;54(6):235-42.
Central administration of perfluorooctanoic
acid inhibits cutaneous inflammation.
Taylor
BK, Kriedt C, Nagalingam S, Dadia N, Badr M.
Department
of Pharmacology, Tulane University Health Sciences Center, New
Orleans, LA 70112, USA.
OBJECTIVE:
To elucidate the site of action of perfluorooctanoic acid (PFOA)
in the carrageenan model of peripheral inflammation.
SUBJECTS: Male Sprague-Dawley rats.
TREATMENT: We first compared the anti-edema effects of systemic
PFOA (50-150 mg/kg) with prototypical nonsteroidal (acetylsalicylic
acid, ASA, 50-200 mg/kg) and steroidal (dexamethasone, 0.5-5.0
mg/kg) drugs after the intraplantar injection of carrageenan
(1%). We then compared the anti-edema effects of systemic PFOA
with local intraplantar (10 mg/kg), and intracerebroventricular
(i.c.v., 0.1-50 mug) routes of administration.
RESULTS: Systemic PFOA was at least as or more efficacious than
ASA or dexamethasone in reducing carrageenan-induced edema.
RU-486 did not change the anti-edema effect of PFOA, ruling
out a contribution of endogenous release of glucorticoids. I.
c. v. PFOA, but not perfluorooctanes, dramatically reduced multiple
signs of inflammation at doses well below the systemically-effective
dose. We conclude that the anti-edema
effect of high systemic doses of PFOA (> or =100 mg/kg, i.
p.) is mediated in part by actions in the brain.
PMID:
15973506 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16040121&query_hl=4
Ecotoxicol
Environ Saf. 2005 Jul 22; [Epub
ahead of print]
The bioconcentration factor of perfluorooctane
sulfonate is significantly larger than that of perfluorooctanoate
in wild turtles (Trachemys scripta elegans and Chinemys reevesii):
An Ai river ecological study in Japan.
Morikawa
A, Kamei N, Harada K, Inoue K, Yoshinaga T, Saito N, Koizumi
A.
Department
of Health and Environmental Sciences, Kyoto University Graduate
School of Medicine, Yoshida Konoe, Sakyo, Kyoto 606-8501, Japan.
Turtles
rank high in the river food chain, and are suitable for predicting
the bioconcentrations of chemicals through the food chain. Trachemys
scripta elegans (N=46) and Chinemys reevesii (N=51) were captured
in a river in Japan, from September to October 2003 and April
to June 2004. Surface water samples were collected simultaneously
from the same sites at which the turtles were caught. Serum
perfluorooctane sulfonate (PFOS) ranged from 2.4 to 486mug/L,
while water PFOS levels ranged from 2.9 to 37ng/L. The geometric
mean (GM) (geometric standard deviation, GSD) of the bioconcentration
factor (BCF) of PFOS was 10,964 (2.5). In contrast, the perfluorooctanoate
(PFOA) level in water ranged from 16.7-87,100ng/L, and serum
PFOA ranged from <0.2 to 870mug/L. The GM (GSD) of the BCF
of PFOA was 3.2 (7.9). Furthermore, the BCF of PFOA decreased
as the PFOA level in the surface water increased. PFOS could
be preferentially bioconcentrated in biota, and PFOA, slightly
bioconcentrated.
PMID:
16040121 [PubMed - as supplied by publisher]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15984763&query_hl=4
Environ Sci Technol. 2005 Jun
1;39(11):3904-10.
Exposure assessment and risk characterization
for perfluorooctanoate in selected consumer articles.
Washburn ST, Bingman TS, Braithwaite
SK, Buck RC, Buxton LW, Clewell HJ, Haroun LA, Kester JE, Rickard
RW, Shipp AM.
ENVIRON International Corporation,
6001 Shellmound Street, Suite 700, Emeryville, California 94608,
USA. swashburn@environcorp.com
An exposure assessment and risk characterization was conducted
to better understand the potential human health significance
of trace levels of perfluorooctanoate (PFO) detected in certain
consumer articles. PFO is the anion of perfluorooctanoic acid
(PFOA). Concentrations of PFO in the consumer articles were
determined from extraction tests and product formulation information.
Potential exposures during consumer use of the articles were
quantified based on an assessment of behavior patterns and regulatory
guidance. Health benchmarks were developed and then compared
to the exposure estimates to yield margins of exposure (MOEs).
A simple one-compartment model was also developed to estimate
contributions of potential consumer exposures to PFO concentrations
in serum. While there are considerable uncertainties in this
assessment, it indicates that exposures to PFO during consumer
use of the articles evaluated in this study are not expected
to cause adverse human health effects in infants, children,
adolescents, adult residents, or professionals nor result in
quantifiable levels of PFO in human serum.
PMID: 15984763 [PubMed - indexed for MEDLINE]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16194675&query_hl=26
Environ
Res. 2005 Oct;99(2):253-61. Epub
2005 Jan 18.
Renal clearance of perfluorooctane sulfonate
and perfluorooctanoate in humans and their species-specific
excretion.
Harada
K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A.
Department
of Health and Environmental Sciences, Kyoto University, Graduate
School of Medicine, Yoshida Konoecho, Kyoto 606-8501, Japan.
Perfluorooctane
sulfonate (PFOS) and perfluorooctanoate (PFOA) are detected
in the environment, as well as more specifically in wildlife
and humans. However, the toxicokinetic aspects of perfluorochemicals
in humans are unclear. In this study, we measured concentrations
of PFOA and PFOS in subjects who had lived in Kyoto city for
more than 10 years. The serum concentrations
of PFOA and PFOS were higher in females who menstruated than
those who did not menstruation (P<0.01), but in males this
did not change by age; the levels in females reached those in
males at an age of 60 years. We then determined the renal
clearances of PFOA and PFOS in young (20-40 years old, N=5 for
each sex) and old (60 years old, N=5 for each sex) subjects
of both sexes. All young females were menstruating, while all
old females were not. The renal clearances were 10(-5)-fold
smaller than the glomerular filtration rate in humans, suggesting
the absence of active excretion in human kidneys. The renal
clearances of PFOA and PFOS were approximately one-fifth of
the total clearance based on their serum half-lives, assuming
a one-compartment model. The sex differences
in renal clearance that have been reported in rats and Japanese
macaques were not found in our human subjects. We tried
to build a one-compartment pharmacokinetic model using the reported
half-lives in human. The model was simple but could predict
the serum concentrations in both males and females fairly well.
We therefore suggest that an internal dose approach using a
pharmacokinetic model should be taken because of the large species
differences in kinetics that exist for PFOA and PFOS.
PMID:
16194675 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15984769&query_hl=13
Environ Sci Technol. 2005 Jun
1;39(11):3946-56.
Quantitative determination of perfluorochemicals
in sediments and domestic sludge.
Higgins CP, Field JA, Criddle CS, Luthy
RG.
Department of Civil and Environmental Engineering, Stanford
University, Stanford, California 94305-4020, USA.
Perfluorochemicals (PFCs) are the subject of increasingly intense
environmental research. Despite their detection both in biota
and in aqueous systems, little attention has been paid to the
possible presence of this class of compounds in solid environmental
matrixes. The limited available data indicate that some PFCs
such as perfluorooctane sulfonate (PFOS) may strongly sorb to
solids, and sewage sludge is widely suspected as a major sink
of PFCs entering municipal waste streams. A quantitative analytical
method was developed that consists of liquid solvent extraction
of the analytes from sediments and sludge, cleanup via solid-phase
extraction, and injection of the extracts with internal standards
into a high-performance liquid chromatography (HPLC) system
coupled to a tandem mass spectrometer (LC/MS/MS). The limits
of detections of the method were analyte and matrix dependent,
but ranged from 0.7 to 2.2 ng/g and 0.041 to 0.246 ng/g (dry
weight) for sludge and sediment, respectively. A demonstration
of the method was performed by conducting a limited survey of
domestic sludge and sediments. The concentration of PFCs in
domestic sludge ranged from 5 to 152 ng/g for total perfluorocarboxylates
and 55 to 3370 ng/g for total perfluoroalkyl sulfonyl-based
chemicals. Data from a survey of San Francisco
Bay Area sediments suggest widespread occurrence of PFCs in
sediments at the low ng/g to sub-ng/g level. Furthermore, substances
that may be transformed to PFOS, such as 2-(N-ethylperfluorooctanesulfonamido)
acetic acid (N-EtFOSAA) and 2-(N-methylperfluorooctanesulfonamido)
acetic acid (N-MeFOSAA), are present in both sediments and sludge
at levels often exceeding PFOS.
PMID: 15984769 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15982707&query_hl=13
Chemosphere. 2005 Jun 24; [Epub
ahead of print]
Evaluation of biochemical effects related
to perfluorooctane sulfonic acid exposure in organohalogen-contaminated
great tit (Parus major) and blue tit (Parus caeruleus) nestlings.
Hoff PT, Van de Vijver K, Dauwe T, Covaci
A, Maervoet J, Eens M, Blust R, De Coen W.
Department of Biology, Research Unit Ecophysiology, Biochemistry
and Toxicology, University of Antwerp, Groenenborgerlaan 171,
B-2020 Antwerp, Belgium.
A perfluorooctane sulfonic acid (PFOS) biomonitoring survey
was conducted on great tit (Parus major) and blue tit (Parus
caeruleus) nestlings from Blokkersdijk, a bird reserve in the
proximity of a fluorochemical plant in Antwerp (Belgium) and
Fort IV, a control area. PFOS, together with 11 organochlorine
pesticides, 20 polychlorinated biphenyl congeners and 7 polybrominated
diphenyl ethers were measured in liver tissue. The hepatic PFOS
concentrations at Blokkersdijk (86-2788 and 317-3322ng/g wet
weight (ww) for great and blue tit, respectively) were among
the highest ever measured and were significantly higher than
at the control area (17-206 and 69-514ng/g ww for great and
blue tit, respectively). The hepatic PFOS
concentration was species- and sex-independent and correlated
significantly and positively with the serum alanine aminotransferase
activity and negatively with the serum cholesterol and
triglyceride levels in both species but did not correlate with
condition or serum protein concentration.
In the great tit, a significant positive correlation was observed
between the liver PFOS concentration and the relative liver
weight. In the blue tit, the hepatic PFOS concentration correlated
positively and significantly with hematocrite values. None of
the investigated organohalogen pollutants except for PFOS were
suggested to be involved in the observed biological alterations.
PMID: 15982707 [PubMed - as supplied by publisher]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15963371&query_hl=13
Environ
Pollut. 2005 Sep;137(2):324-33.
Perfluorooctane sulfonic acid and organohalogen
pollutants in liver of three freshwater fish species in Flanders
(Belgium): relationships with biochemical and organismal effects.
Hoff
PT, Van Campenhout K, Van de Vijver K, Covaci A, Bervoets L,
Moens L, Huyskens G, Goemans G, Belpaire C, Blust R, De Coen
W.
Department
of Biology, Research Unit Ecophysiology, Biochemistry and Toxicology,
Antwerp University, Groenenborgerlaan 171, B-2020 Antwerp, Belgium.
A
perfluorooctane sulfonic acid (PFOS) assessment was conducted
on gibel carp (Carassius auratus gibelio), carp (Cyprinus carpio),
and eel (Anguilla anguilla) in Flanders (Belgium). The
liver PFOS concentrations in fish from the Ieperlee canal
(Boezinge, 250-9031 ng/g wet weight, respectively) and
the Blokkersdijk pond (Antwerp, 633-1822 ng/g wet weight)
were higher than at the Zuun basin (Sint-Pieters-Leeuw, 11.2-162
ng/g wet weight) and among the highest
in feral fish worldwide. Eel from the Oude Maas pond
(Dilsen-Stokkem) and Watersportbaan basin (Ghent) had PFOS concentrations
ranging between 212 and 857 ng/g wet weight. The hepatic PFOS
concentration was significantly and positively related with
the serum alanine aminotransferase activity, and negatively
with the serum protein content in eel and carp. The hepatic
PFOS concentration in carp correlated significantly and negatively
with the serum electrolyte concentrations whereas a significant
positive relation was found with the hematocrit in eel. Although
13 organochlorine pesticides, 22 polychlorinated biphenyl (PCB)
congeners and 7 polybrominated diphenyl ethers (PBDEs) were
also measured in the liver tissue, only PCB 28, PCB 74, gamma-hexachlorocyclohexane
(gamma-HCH) and hexachlorobenzene (HCB) were suggested to contribute
to the observed serological alterations in eel.
PMID:
15963371 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15896450&query_hl=33
Regul
Toxicol Pharmacol. 2005 Jun;42(1):145.
Epub 2005 Feb 24.
Human health risks from exposures to
perfluorooctanoic acid: A critique of Butenhoff et al. 2004.
Kropp
T, Houlihan J.
Environmental
Working Group, Toxics Division, 1436 U St NW, STE 100, Washington,
DC 20009, USA.
Publication
Types:
• Letter
PMID:
15896450 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15926594&query_hl=28
Environ Sci Technol. 2005 May
1;39(9):3388-92.
Sonochemical decomposition of perfluorooctane
sulfonate and perfluorooctanoic acid.
Moriwaki H, Takagi Y, Tanaka M, Tsuruho
K, Okitsu K, Maeda Y.
Osaka City Institute of Public Health & Environmental Sciences,
8-34, Tojo-cho, Tennoji-ku, Osaka 543-0026, Japan. hiroshi.moriwaki@iphes.city.osaka.jp
Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid
(PFOA) are shown to be globally distributed, environmentally
persistent, and bioaccumulative. Although the toxicities of
these compounds were reported, the cleanup procedure from the
environment is not developed because of their inertness. In
this report the sonochemical degradations of PFOS and PFOA to
the products through the fission of the perfluorocarbon chains
were observed and the half-life times of the PFOS and PFOA degradations
under an argon atmosphere determined to be 43 and 22 min, respectively.
The shortening of perfluorocarbon chain
of PFOS and PFOA leads to the lowering of the toxicity in view
of the decrease of the persistence, and the technique would
contribute to the remediation of the environmental pollution
by these compounds.
PMID: 15926594 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15865257&query_hl=21
Drug Chem Toxicol. 2005;28(2):135-58.
Subchronic toxicity of a fluoroalkylethanol
mixture in rats.
Ladics GS, Stadler JC, Makovec GT, Everds
NE, Buck RC.
DuPont Company Haskell Laboratory
for Health and Environmental Sciences, Newark, Delaware 19714,
USA. Gregory.S.Ladics@usa.dupont.com
The objective of this study was to evaluate the subchronic
toxicity of a commercial fluoroalkylethanol mixture, which is
an intermediate in the production of fluoroorganic compounds
that are used as protectants and surfactants. The test substance
was administered daily by gavage to Sprague-Dawley rats as a
suspension in aqueous methylcellulose. The dosages were 0, 25,
100, or 250 mg kg(-1) day(-1). A 1- and 3-month recovery period
was included to evaluate the reversibility of toxic effects.
No test substance-related mortality or neurotoxicity occurred.
Body weights and/or nutritional parameters were significantly
reduced at 100 and 250 mg kg(-1) day(-1), and these effects
were reversible. Broken and absent teeth
were observed in rats dosed with 250 mg kg(-1) day(-1), and
microscopic tooth lesions (ameloblast degeneration/disorganization)
occurred at 100 and 250 mg kg(-1) day(-1) and persisted
with decreased severity throughout recovery. Decreased red cell
mass parameters occurred at 90 days in the 250 mg kg(-1) day(-1)
group, but red cell counts were normal thereafter during recovery.
A persistent elevation of liver weights was seen in groups given
> or =100 mg kg(-l) day(-1). The increased weights correlated
with microscopic hepatocellular hypertrophy only in males and
females administered 250 mg kg(-1) day(-1). Hepatic beta-oxidation
was increased in a dose-dependent manner and persisted through
1 month of recovery at 250 mg kg(-1) day(-1). Increased kidney
weights were observed at 25 (females only), 100, and 250 mg
kg(-1) day(-1). These elevated weights persisted in the high
dose after recovery and correlated with microscopic tubular
hypertrophy (males only). Thyroid follicular hypertrophy was
present at 100 and 250 mg kg(-1) day(-1) but was not present
after recovery. Total fluorine in whole
blood increased with continuous dosing and achieved steady state
in approximately 42 days. Both plasma and urine fluoride levels
were elevated in a dose-dependent manner. Under the conditions
of the study, the no-observed adverse effect level for this
mixture was 25 mg kg(-1) day(-1) for subchronic toxicity.
PMID: 15865257 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15913661&query_hl=4
Mar
Pollut Bull. 2005 May 20; [Epub
ahead of print]
A global survey of perfluorinated acids
in oceans.
Yamashita
N, Kannan K, Taniyasu S, Horii Y, Petrick G, Gamo T.
National
Institute of Advanced Industrial Science and Technology (AIST),
16-1, Onogawa, Tsukuba, Ibaraki 305-8569, Japan.
Perfluorinated
acids and their salts have emerged as an important class of
global environmental contaminants. Biological monitoring surveys
conducted using tissues of marine organisms reported the occurrence
of perfluorooctanesulfonate (PFOS) and related perfluorinated
compounds in biota from various seas and oceans, including the
Arctic and the Antarctic Oceans. Occurrence of perfluorinated
compounds in remote marine locations is of concern and indicates
the need for studies to trace sources and pathways of these
compounds to the oceans. Determination of sub-parts-per-trillion
(ng/L) or parts-per-quadrillion (pg/L) concentrations of aqueous
media has been impeded by relatively high background levels
arising from procedural or instrumental blanks. Our research
group has developed a reliable and highly sensitive analytical
method by which to monitor perfluorinated compounds in oceanic
waters. The method developed is capable of detecting PFOS, perfluorohexanesulfonate
(PFHS), perfluorobutanesulfonate (PFBS), perfluorooctanoate
(PFOA), perfluorononanoate (PFNA), and perfluorooctanesulfonamide
(PFOSA) at a few pg/L in oceanic waters. The method was applied
to seawater samples collected during several international research
cruises undertaken during 2002-2004 in the central to eastern
Pacific Ocean (19 locations), South China Sea and Sulu Seas
(five), north and mid Atlantic Ocean (12), and the Labrador
Sea (20). An additional 50 samples of coastal seawater from
several Asian countries (Japan, China, Korea) were analyzed.
PFOA was found at levels ranging from
several thousands of pg/L in water samples collected from coastal
areas in Japan to a few tens of pg/L in the central Pacific
Ocean. PFOA was the major contaminant detected in oceanic waters,
followed by PFOS. Further studies are being conducted
to elucidate the distribution and fate of perfluorinated acids
in oceans.
PMID:
15913661 [PubMed - as supplied by publisher]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15888668&query_hl=4
Toxicol Sci. 2005 May 11; [Epub
ahead of print]
Induction of hepatic peroxisome proliferation
by 8-2 telomer alcohol feeding in mice: Formation of perfluorooctanoic
acid in the liver.
Kudo N, Iwase Y, Okayachi H, Yamakawa
Y, Kawashima Y.
Faculty of Pharmaceutical Sciences, Josai University, Keyakidai
1-1, Sakado, Saitama 350-0295, Japan.
The effects of dietary administration of 1H, 1H, 2H, 2H-perfluorodecanol
(8-2 telomer alcohol), on peroxisome proliferation in the liver
of mice were studied. Male ddY mice were fed on a diet containing
8-2 telomer alcohol at concentrations of 0, 0.025, 0.05, 0.1
and 0.2% (w/w) for 7, 14, 21 and 28 days. These treatments with
8-2 telomer alcohol caused liver enlargement in a dose- and
duration-dependent manner. Peroxisome proliferation in the liver
of mice was confirmed by electron microscopic examination. Peroxisomal
acyl-CoA oxidase was induced by these treatments with 8-2 telomer
alcohol in a dose- and time-dependent manner. The concentration
of perfluorooctanoic acid (PFOA) and related compounds were
determined in the liver and plasma, since PFOA had been shown
to be a possible metabolite of 8-2 telomer alcohol and to cause
significant peroxisome proliferation in rodents. Five metabolites,
namely, perfluorooctanoic acid (PFOA), perfluorononanoic acid
(PFNA), 2H, 2H-perfluorodecanoic acid (8-2 telomer acid), and
two unidentified metabolites, were present in the liver and
serum. PFOA was confirmed to be accumulated in the liver of
mice following the administration of 8-2 telomer alcohol in
a dose- and duration-dependent manner.
A linear relationship was observed between the concentration
of PFOA and the activity of peroxisomal acyl-CoA oxidase in
the liver of mice. These results strongly suggest that PFOA,
but not 8-2 telomer alcohol itself, caused peroxisome proliferation
in the liver. The present study provided evidence that 8-2 telomer
alcohol is converted into PFOA in vivo and that the PFOA formed
produces biological effects in the liver of mice.
PMID: 15888668 [PubMed - as supplied by publisher]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16110997&query_hl=5
Environ Toxicol Chem. 2005 May;24(5):1172-81.
Short-term exposures of fish to perfluorooctane
sulfonate: acute effects on fatty acyl-coa oxidase activity,
oxidative stress, and circulating sex steroids.
Oakes KD, Sibley PK, Martin JW, MacLean
DD, Solomon KR, Mabury SA, Van Der Kraak GJ.
Department of Zoology, University of Guelph, Guelph, Ontario
N1G 2W1, Canada.
This study investigated the effects of exposure to waterborne
perfluorooctane sulfonate (PFOS) on oxidative stress and reproductive
endpoints in fish. Exposures utilized species commonly used
in toxicological testing, including the fathead minnow (Pimephales
promelas) and rainbow trout (Oncorhynchus mykiss), as well as
relatively insensitive taxa such as creek chub (Semotilus atromaculatus),
spottail shiner (Notropis hudsonius), and white sucker (Catostomus
commersoni). In all fish species, short-term (14-28 d) exposure
to PFOS produced only modest mortality at concentrations consistent
with environmental spill scenarios. However, PFOS
consistently increased hepatic fatty acyl-CoA oxidase activity
and increased oxidative damage, as quantified using the 2-thiobarbituric
acid-reactive substances assay. Plasma testosterone, 11-ketotestosterone,
and 17beta-estradiol titers were often elevated with PFOS exposure.
Vitellogenin, the egg yolk precursor protein, was occasionally
altered in the plasma with PFOS exposure, but responses varied
with maturity. Oviposition frequency and egg deposition
in fathead minnow were not significantly impaired with PFOS
exposure, despite a trend toward progressive impairment with
increasing exposure concentrations. Although
short-term PFOS exposure produced significant impacts on biochemical
and reproductive endpoints in fish at concentrations consistent
with environmental spills, the impact of long-term exposure
to environmentally relevant concentrations of PFOS is unclear.
PMID: 16110997 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15883668&query_hl=4
Arch Environ Contam Toxicol. 2005
May;48(4):559-66.
Perfluorinated compounds in aquatic organisms
at various trophic levels in a great lakes food chain.
Kannan K, Tao L, Sinclair E, Pastva SD,
Jude DJ, Giesy JP.
Wadsworth Center, New York State Department of Health and Department
of Environmental Health and Toxicology, School of Public Health,
State University of New York at Albany, Empire State Plaza,
509, Albany, New York, 12201-0509, USA, kkannan@wadsworth.org.
Trophic transfer of perfluorooctanesulfonate (PFOS) and other
related perfluorinated compounds was examined in a Great Lakes
benthic foodweb including water-algae-zebra mussel-round goby-smallmouth
bass. In addition, perfluorinated compounds were measured in
livers and eggs of Chinook salmon and lake whitefish, in muscle
tissue of carp, and in eggs of brown trout collected from Michigan.
Similarly, green frog livers, snapping turtle plasma, mink livers,
and bald eagle tissues were analyzed to determine concentrations
in higher trophic-level organisms in the food chain.
PFOS was the most widely detected compound in benthic organisms
at various trophic levels. Concentrations of PFOS in benthic
invertebrates such as amphipods and zebra mussels were approximately
1000-fold greater than those in surrounding water, which suggested
a bioconcentration factor (BCF; concentration in biota/concentration
in water) of 1000 in benthic invertebrates. Concentrations of
PFOS in round gobies were two- to fourfold greater than those
in their prey organisms such as zebra mussels and amphipods.
Concentrations of PFOS in predatory fishes (Chinook salmon and
lake whitefish) were 10 to 20-fold greater than those in their
prey species. Concentrations of PFOS in mink and bald eagles
were, on average, 5- to 10-fold greater than those in Chinook
salmon, carp, or snapping turtles. Because of the accumulation
of PFOS in liver and blood, the biomagnification factor (BMF)
of perfluorinated compounds in higher trophic-level organisms
such as salmonid fishes, mink, and eagles were based on the
concentrations in livers or plasma. Overall, these results suggest
a BCF of PFOS of approximately 1000 (whole-body based) in benthic
invertebrates, and a BMF of 10 to 20 in mink or bald eagles,
relative to their prey items. Eggs of
fish contained notable concentrations of PFOS, suggesting oviparous
transfer of this compound. PFOA was found in water, but its
biomagnification potential was lower than that of PFOS.
PMID: 15883668 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15871280&query_hl=4
Environ Sci Technol. 2005 Apr
1;39(7):2383-8.
Efficient decomposition of environmentally
persistent perfluorocarboxylic acids by use of persulfate as
a photochemical oxidant.
Hori H, Yamamoto A, Hayakawa E, Taniyasu
S, Yamashita N, Kutsuna S, Kiatagawa H, Arakawa R.
National Institute of Advanced Industrial Science and Technology,
AIST Tsukuba West, 16-1 Onogawa, Tsukuba 305-8569, Japan. h-hori@aist.go.jp
Photochemical decomposition of persistent perfluorocarboxylic
acids (PFCAs) in water by use of persulfate ion (S2O8(2-)) was
examined to develop a technique to neutralize stationary sources
of PFCAs. Photolysis of S2O8(2-) produced highly oxidative sulfate
radical anions (SO4-), which efficiently
decomposed perfluorooctanoic acid (PFOA) and other PFCAs bearing
C4-C8 perfluoroalkyl groups. The major products were F- and
CO2; also, small amounts of PFCAs with shorter than initial
chain lengths were detected in the reaction solution.
PFOA at a concentration of 1.35 mM (typical
of that in untreated wastewater after an emulsifying process
in fluoropolymer manufacture) was completely decomposed
by a photochemical system with 50 mM S2O8(2-) and 4 h of irradiation
from a 200-W xenon-mercury lamp. The initial
PFOA decomposition rate was 11 times higher than with photolysis
alone. All sulfur-containing species in the reaction
solution were eventually transformed to sulfate ions by this
method. This method was successfully applied to the decomposition
of perfluorononanoic acid contained in a floor wax solution.
PMID: 15871280 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15866760&query_hl=4
Environ Health Perspect. 2005 May;113(5):539-45.
Historical comparison of perfluorooctanesulfonate,
perfluorooctanoate, and other fluorochemicals in human blood.
Olsen GW, Huang HY, Helzlsouer KJ, Hansen
KJ, Butenhoff JL, Mandel JH.
Medical Department, 3M Company,
Mail Stop 220-6W-08, St. Paul, MN 55144, USA. gwolsen@mmm.com
The purpose of this investigation was to determine whether
there has been a change in the human blood concentration of
perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA),
and five other fluorochemicals since 1974. Blood samples were
collected in 1974 (serum) and 1989 (plasma) from volunteer participants
of a large community health study. The study included a total
of 356 samples (178 from each time period). These samples were
analyzed by high-pressure liquid chromatography/tandem mass
spectrometry methods. The median 1974 and 1989 fluorochemical
concentrations, respectively, were as follows: PFOS, 29.5 ng/mL
vs. 34.7 ng/mL; PFOA, 2.3 ng/mL vs. 5.6 ng/mL; perfluorohexanesulfonate
(PFHS), 1.6 ng/mL vs. 2.4 ng/mL; and N-ethyl perfluorooctanesulfonamidoacetate
(PFOSAA), less than the lower limit of quantitation (LLOQ; 1.6
ng/mL, vs. 3.4 ng/mL). For N-methyl perfluorooctanesulfonamidoacetate
(M570), perfluorooctanesulfonamide, and perfluorooctanesulfonamidoacetate,
median serum concentrations in both years were less than the
LLOQ values (1.0, 1.0, and 2.5 ng/mL, respectively). Statistical
analysis of 58 paired samples indicated that serum concentrations
of PFOS, PFOSAA, PFOA, PFHS, and M570 were significantly (p
< 0.001) higher in 1989 than in 1974. The data from
1989 were then compared with geometric mean fluorochemical concentrations
of serum samples collected in 2001 from 108 American Red Cross
adult blood donors from the same region. Except for M570, there
were no statistically significant (p < 0.05) geometric mean
fluorochemical concentration differences between the 1989 and
2001 samples. In conclusion, based on this study population,
PFOS and other serum fluorochemical concentrations have increased
between 1974 and 1989. Comparison with other regional data collected
in 2001 did not suggest a continued increase in concentrations
since 1989.
PMID: 15866760 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15865261&query_hl=4
Drug Chem Toxicol. 2005;28(2):197-209.
Subcellular distribution and protein
binding of perfluorooctanoic acid in rat liver and kidney.
Han X, Kemper RA, Jepson GW.
DuPont Haskell Laboratory for
Health and Environmental Sciences, Newark, Delaware 19714, USA.
xing.han@usa.dupont.com
Perfluorooctanoic acid (PFOA) is an organic
fluorochemical, and its elimination in rats is markedly sex-dependent.
Liver and kidney are two primary tissues of distribution of
PFOA in rats. In this study, the subcellular distribution
of PFOA in male and female rat liver and kidney was examined.
The results demonstrated that PFOA content
in the liver cytosol of the female rat was significantly higher
(49 +/- 6% of total radioactive residues, TRR) than in the male
liver (26 +/- 5% TRR), whereas PFOA distribution in the
heavier subcellular fractions, especially the nuclei and cell
debris fraction, was marginally higher in male rat liver. In
rat kidney, more than 70% of PFOA was distributed in the cytosolic
fraction, with no significant difference between sexes. The
degree of protein binding of PFOA in rat liver and kidney cytosol
was analyzed by two different chromatographic methods. The percentage
of protein-bound PFOA in the liver cytosol was found to be approximately
55% in both male and female rats. In contrast, significantly
more PFOA was bound to cytosolic proteins in the kidney of male
rats (42 +/- 6% TRR) than in females (17 +/- 5% TRR).
Ligand blotting analysis revealed that multiple proteins from
the liver cytosol, nuclei, and mitochondria fractions were capable
of specific binding to PFOA.
PMID: 15865261 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16124282&query_hl=5
Environ Sci Technol. 2005 Aug 1;39(15):5517-23.
Circumpolar study of perfluoroalkyl
contaminants in polar bears (Ursus maritimus).
Smithwick M, Mabury SA, Solomon KR, Sonne
C, Martin JW, Born EW, Dietz R, Derocher AE, Letcher RJ, Evans
TJ, Gabrielsen GW, Nagy J, Stirling I, Taylor MK, Muir DC.
Department of Environmental Biology, University of Guelph,
Bovey Building, Guelph, Ontario N1G 2W1, Canada.
Perfluoroalkyl substances were determined in liver tissues
and blood of polar bears (Ursus maritimus) from five locations
in the North American Arctic and two locations in the European
Arctic. Concentrations of perfluorooctane sulfonate (PFOS),
perfluorohexane sulfonate, heptadecafluorooctane sulfonamide,
and perfluoroalkyl carboxylates with C(8)-C(15) perfluorinated
carbon chains were determined using liquid chromatography tandem
mass spectrometry. PFOS concentrations
were significantly correlated with age at four of seven sampling
locations, while gender was not correlated to concentration
for any compound measured. Populations
in South Hudson Bay (2000-2730 ng/g wet wt), East Greenland
(911-2140 ng/g wet wt), and Svalbard (756-1290 ng/g wet wt)
had significantly (P < 0.05) higher PFOS concentrations than
western populations such as the Chukchi Sea (435-729 ng/g wet
wt). Concentrations of perfluorocarboxylic acids (PFCAs)
with adjacent chain lengths (i.e., C9:C10 and C10:C11) were
significantly correlated (P < 0.05), suggesting PFCAs have
a common source within a location, but there were differences
in proportions of PFCAs between eastern and western location
sources. Concentrations of PFOS in liver
tissue at five locations were correlated with concentrations
of four polychlorinated biphenyl congeners (180, 153, 138, and
99) in adipose tissue of bears in the same populations, suggesting
similar transport pathways and source regions of PFOS or precursors.
PMID: 16124282 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15839574&query_hl=4
Environ Toxicol Chem. 2005 Apr;24(4):981-6.
Perflouroalkyl contaminants in liver
tissue from East Greenland polar bears (Ursus maritimus).
Smithwick M, Muir DC, Mabury SA, Solomon
KR, Martin JW, Sonne C, Born EW, Letcher RJ, Dietz R.
Department of Environmental Biology, University of Guelph,
Guelph, Ontario N1G 2W1, Canada.
Perfluoroalkyl substances were determined in polar bears (Ursus
maritimus) collected in East Greenland (69 degrees 00'N to 74
degrees 00"N) to compare with other populations and to
examine effects of age and gender on concentrations of these
contaminants. Hepatic tissue (n = 29) was analyzed for perfluorooctane
sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexane
sulfonate, heptadecafluorooctane sulfonamide (PFOSA), and perfluoroalkyl
carboxylates (PFCAs) with C9-C15 perfluorinated carbon chains
by liquid chromatography tandem mass spectrometry. Concentrations
of PFOS found in samples from East Greenland (mean = 2,470+/-1,320
ng/g wet weight) were similar to Hudson Bay, Canada, and both
populations had significantly greater concentrations than those
reported for Alaska, suggesting a spatial trend. Male
bears showed a significant increase in concentration up to age
six for PFCAs with C10-C14 carbon chains (r2 > or = 0.50,
p < or = 0.05). Significant correlations were found between
adjacent chain length PFCAs, (e.g., PFNA to PFDA: p < 0.05;
r2 = 0.90). This may indicate a common source for these chemicals,
although the specifics of source and mode of transport are unknown.
No significant correlations were found between concentrations
of PFCAs in liver tissue and previously reported polychlorinated
biphenyl (PCB) congeners analyzed in fat samples from the same
bears.
PMID: 15839574 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15862018
Wei
Sheng Yan Jiu. 2005 Jan;34(1):37-9.
[Effects of perfluorooctane sulfonate
on spermiogenesis function of male rats]
[Article
in Chinese]
Fan
YO, Jin YH, Ma YX, Zhang YH.
School
of Public Health, China Medical University, Shenyang 110001,
China.
OBJECTIVE:
To evaluate the effects of administration of perfluorooctane
sulfonate (PFOS) on spermiogenesis
function of male rats.
METHODS: 36 male rats were randomly divided into 4 groups, which
received 0, 0.5, 1.5, 4.5 mg x kg(-1) PFOS by food intake per
day for 65 days. The testicular and epididymal viscera coefficients,
the number, motility and deformity of sperm were examined. The
activities of lactate dehydrogenase isoenzyme-x (LDHx), sorbitol
dehydrogenase (SDH) and the generation of maglonydiadehyde (MDA)
in the testes were also measured.
RESULTS: The viscera coefficients did not show any significant
change ( P > 0.05) while the body weight and weight of testis
decreased ( P < 0.05) in treated rats compared with the corresponding
control group animals. In 1.5,4.5 mg x kg(-1) PFOS treated rats
there were significant decreases in the
sperm count (P < 0.05) and the mean activities of LDHx and
SDH whereas obvious increases in the rate of sperm deformity
( P < 0.05). In 4.5 mg x kg(-1) PFOS group the generation
of MDA increased (P < 0.05) while the motility of sperm reduced
(P < 0.05) with respect to the control value.
CONCLUSION: It suggested that PFOS could elicit the impairment
of sperm production and maturation of male rats.
PMID:
15862018 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15798805
J Environ Monit. 2005 Apr;7(4):371-7. Epub 2005
Mar 7.
Perfluorinated organic compounds in human
blood serum and seminal plasma: a study of urban and rural tea
worker populations in Sri Lanka.
Guruge KS, Taniyasu S, Yamashita N, Wijeratna
S, Mohotti KM, Seneviratne HR, Kannan K, Yamanaka N, Miyazaki
S.
Toxico-Biochemistry Section, National Institute of Animal Health,
Kannondai 3-1-5, Tsukuba, Ibaraki 305-0856, Japan. guruge@affrc.go.jp
Concentrations and accumulation of 13 fluorinated organic compounds
(FOCs) in human sera and seminal
plasma were measured in an Asian developing country, Sri Lanka.
Six of the FOCs, PFOS (perfluorooctanesulfonate),
PFHS (perfluorohexanesulfonate),
PFUnA (perfluoroundecanoic acid),
PFDA (perfluorodecanoic acid),
PFNA (perfluorononanoic acid) and
PFOA (perfluorooctanoic acid),
were detected in all of the sera samples. Measurable
quantities of two main perfluorosulfonates, PFOS
and PFHS, were found in
all seminal plasma samples. The detection frequency of the predominant
perfluoroalkylcarboxylate, PFOA,
in seminal plasma was >70%. Accumulation of PFOS
in sera was significantly positively correlated with PFOA,
PFHS and PFNA. Positive linear regressions were
also found between PFNA and PFUnA and PFNA and PFDA suggesting
that these compounds may have a similar origin of exposure and
accumulation. Significantly positive associations were
observed for partitioning of both PFOS and PFNA between sera
and seminal plasma. The accumulation of FOCs was not significantly
different in sera from Colombo (urban population) and Talawakele
(rural conventional tea workers). However, the Haldummulla population
(rural organic tea workers) had relatively lower exposure to
FOCs compared to the other two groups, urban and rural conventional
tea workers. Concentrations of FOCs in
Sri Lanka were similar to those reported for industrialized
countries suggesting that human exposure to such chemicals is
widespread even in developing countries. The novel finding of
FOCs in human seminal plasma implies that further studies are
needed to determine whether long-term exposure in humans can
result in reproductive impairments.
PMID: 15798805 [PubMed - in process]
Toxicology - Volume 211, Issues 1-2 , 1 July 2005, Pages 139-148
Perfluorooctanoate: Placental and lactational
transport pharmacokinetics in rats
Paul M. Hinderliter (a), Eve Mylchreest (a), Shawn A. Gannon
(a), John L. Butenhoff (b) and Gerald L. Kennedy, Jr.(a)
(a) DuPont Haskell Laboratory for
Health and Environmental Sciences, 1090 Elkton Road, P.O. Box
50, Newark, DE 19714, USA
(b) 3M Company, Medical Department,
3M Center 220-06-W-08, St. Paul, MN 55144, USA
This study was conducted to develop a quantitative understanding
of the potential for gestational and lactational transfer of
perfluorooctanoate (PFOA) in the rat. Time-mated female rats
were dosed by oral gavage once daily at concentrations of 3,
10, or 30 mg/kg/day of the ammonium salt of PFOA (APFO)
starting on gestation (G) day 4 and continuing until sacrifice.
On days 10, 15, and 21G, five rats per dose level were sacrificed
and blood samples were collected 2 h post-dose. Embryos
were collected on day 10G, amniotic fluid, placentas, and embryos/fetuses
were collected on days 15 and 21G, and fetal blood samples were
collected on day 21G. Five rats per dose level were allowed
to deliver and nurse their litters, and on days 3, 7, 14, and
21 post-partum (PP) milk and blood samples of maternal and pup
were collected 2 h post-dose. All samples were analyzed
by high-performance liquid chromatography–mass spectrometry
(HPLC–MS) for PFOA concentration. Concentrations of PFOA
in maternal plasma and milk attained steady state during the
sampling interval. The steady-state concentrations in maternal
plasma were 10–15, 25–30, and 60–75 ?g/mL
in rats receiving 3, 10, and 30 mg/kg, respectively. Steady-state
concentrations in milk were approximately 10 times less than
those in maternal plasma. The concentration
of PFOA in fetal plasma on day 21G was approximately half the
steady-state concentration in maternal plasma. The milk concentrations
appeared to be generally comparable to the concentrations in
pup plasma. Pup plasma concentrations decreased from
day 3PP to day 7PP, and were similar on days 7, 14, and 21PP
at all dose levels. PFOA was detected
in placenta (days 15 and 21G), amniotic fluid (days 15 and 21G),
embryo (days 10 and 15G), and fetus (day 21G). These
pharmacokinetics allow estimation of the dose to developing
and nursing rat offspring following maternal exposure.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15768499
Bull Environ Contam Toxicol. 2005
Jan;74(1):64-9.
Airborne perfluorooctanoate may be a substantial source contamination
in Kyoto area, Japan.
Harada K, Nakanishi S, Saito N, Tsutsui
T, Koizumi A.
Department of Health and Environmental Sciences, Kyoto University
School of Public Health, Kyoto, Japan.
No abstract available
PMID: 15768499 [PubMed - indexed for MEDLINE]
Environmental
Pollution Volume 136, Issue 2 , July 2005,
Pages 323-329
Preliminary screening of perfluorooctane sulfonate (PFOS)
and other fluorochemicals in fish, birds and marine mammals from
Greenland and the Faroe Islands
Rossana
Bossia (a), Frank F. Riget (a), Rune Dietz (a), Christian Sonne
(a), Patrik Fauser (a), Maria Dam (b)and Katrin Vorkamp (a)
(a)
National Environmental Research Institute, Frederiksborgvej
399, 4000-Roskilde, Denmark
(b) Food and Environmental Agency, Thorshavn, Faroe Islands
Extensive screening analyses of perfluorooctane sulfonate
(PFOS) and related perfluorinated compounds in biota samples
from all over the world have identified PFOS as a global pollutant
and have shown its bioaccumulation into higher trophic levels
in the food chain. Perfluorinated compounds have been found
in remote areas as the Arctic. In this study a preliminary screening
of PFOS and related compounds has been performed in liver samples
of fish, birds and marine mammals from Greenland and the Faroe
Islands. PFOS was the predominant fluorochemical
in the biota analyzed, followed by perfluorooctane sulfonamide
(PFOSA). PFOS was found at concentrations above LOQ (10 ng/g
wet weight) in 13 out of 16 samples from Greenland and in all
samples from the Faroe Islands. The results from Greenland
showed a biomagnification of PFOS along the marine food chain
(shorthorn sculpin<ringed seal<polar bear).
The greatest concentration of PFOS was found in liver of polar
bear from east Greenland (mean: 1285 ng/g wet weight, n=2).
The geographical distribution of perfluorinated
compounds in Greenland was similar to that of persistent organohalogenated
compounds (OHCs), with the highest concentrations in east Greenland,
indicating a similar geographical distribution to that of OHCs,
with higher concentrations in east Greenland than in west Greenland.
Perfluorinated acids were detected in livers of fish, birds
and marine mammals from Greenland and the Faroe Islands.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15779762
Environ
Toxicol Chem. 2005 Mar;24(3):617-28.
Effects of contaminant exposure on reproductive
success of ospreys (Pandion haliaetus) nesting in Delaware River
and Bay, USA.
Toschik
PC, Rattner BA, McGowan PC, Christman MC, Carter DB, Hale RC,
Matson CW, Ottinger MA.
Marine,
Estuarine, and Environmental Science Program, University of
Maryland, College Park, Maryland 20742, USA.
Despite
serious water-quality problems and pollutant loading and retention,
Delaware River and Bay (USA) provide important wildlife habitat.
In 2002, we conducted a comprehensive evaluation of contaminant
exposure and reproduction of ospreys (Pandion haliaetus) breeding
in Delaware River and Bay. Sample eggs were collected from 39
nests and analyzed for organochlorine pesticides, polychlorinated
biphenyls (PCBs), and mercury; a subset of 15 eggs was analyzed
for perfluorinated compounds and polybrominated diphenyl ethers
(PBDEs). The fate of each nest was monitored weekly. Concentrations
of 10 organochlorine pesticides or metabolites, total PCBs,
and several toxic PCB congeners were greater (p < 0.05) in
eggs collected between the Chesapeake and Delaware Canal (C
and D Canal) and Trenton (Delaware River and northern Bay) compared
to other sites. Concentrations of p,p'-dichlorodiphenyldichloroethylene
(p,p'-DDE; 0.785-3.84 microg/g wet wt) and total PCBs (5.50-14.5
microg/g wet wt) in eggs collected between the C and D Canal
and Trenton were similar to levels recently found in the Chesapeake
Bay. In all study segments, at least one young fledged from
66 to 75% of nests. Productivity for Delaware Inland Bays (reference
area) and southern Delaware Bay was 1.17 and 1.42 fledglings/active
nest, respectively; north of the C and D Canal, productivity
was 1.00 fledgling/active nest, which is marginally adequate
to maintain the population. Using these data, a logistic regression
model found that contaminant concentrations (p,p'-DDE, heptachlor
epoxide, chlordane and metabolites, and total PCBs) were predictive
of hatching success. Several perfluorinated
compounds and PBDEs were detected in eggs at concentrations
approaching 1 microg/g wet weight. These findings provide
evidence that contaminants continue to be a significant stressor
on osprey productivity in the northern Delaware River and Bay.
PMID:
15779762 [PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15768565
Artif
Cells Blood Substit Immobil Biotechnol. 2005;33(1):47-63.
Understanding the fundamentals of perfluorocarbons
and perfluorocarbon emulsions relevant to in vivo oxygen delivery.
Riess
JG.
MRI
Institute, University of California at San Diego, San Diego,
California, USA. jriess@allp.com
The
unique behavior of perfluorocarbons (PFCs), including their
high oxygen dissolving capacity, hydrophobic and lipophobic
character, and extreme inertness, derive directly, in a predictable
manner, from the electronic structure and spatial requirements
of the fluorine atom. Their
low water solubility is key to the prolonged in vivo persistence
of the now commercially available injectable microbubbles that
serve as contrast agents for diagnostic ultrasound imaging.
Oxygent, a stable, small-sized emulsion of a slightly
lipophilic, rapidly excreted PFC, perfluorooctyl bromide (perflubron),
has been engineered. Significant oxygen delivery has been established
in animal models and through Phase II and III human clinical
trials. However, an inappropriate testing
protocol and the lack of funding led to temporary suspension
of the trials.
PMID:
15768565 [PubMed - in process]
From
Science Direct
Environmental Pollution
- Article in Press, Corrected Proof. Available
online 5 March 2005.
Preliminary
screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals
in fish, birds and marine mammals from Greenland and the Faroe
Islands
Rossana
Bossia (a), Frank F. Riget (a), Rune Dietz (a), Christian Sonne
(a), Patrik Fauser (a), Maria Dam (b) and Katrin Vorkamp (a)
(a) National Environmental
Research Institute, Frederiksborgvej 399, 4000-Roskilde, Denmark
(b) Food and Environmental Agency, Thorshavn, Faroe Islands
Extensive screening
analyses of perfluorooctane sulfonate (PFOS) and related perfluorinated
compounds in biota samples from all over the world have identified
PFOS as a global pollutant and
have shown its bioaccumulation into higher trophic levels in
the food chain. Perfluorinated compounds have been found in
remote areas as the Arctic. In this study a preliminary screening
of PFOS and related compounds has been performed in liver samples
of fish, birds and marine mammals from Greenland and the Faroe
Islands. PFOS was the predominant fluorochemical
in the biota analyzed, followed by perfluorooctane sulfonamide
(PFOSA). PFOS was found at concentrations above LOQ (10 ng/g
wet weight) in 13 out of 16 samples from Greenland and in all
samples from the Faroe Islands. The results from Greenland showed
a biomagnification of PFOS along the marine food chain (shorthorn
sculpin<ringed seal<polar bear). The greatest concentration
of PFOS was found in liver of polar bear from east Greenland
(mean: 1285 ng/g wet weight, n=2). The geographical distribution
of perfluorinated compounds in Greenland was similar to that
of persistent organohalogenated compounds (OHCs), with the highest
concentrations in east Greenland, indicating a similar geographical
distribution to that of OHCs, with higher concentrations in
east Greenland than in west Greenland. Perfluorinated acids
were detected in livers of fish, birds and marine mammals from
Greenland and the Faroe Islands.
From
Science Direct
Biochemical and Biophysical
Research Communications - Volume 329, Issue 2 , 8 April 2005,
Pages 487-494
Effects of PFOS and PFOA on L-type Ca2+ currents in
guinea-pig ventricular myocytes
Kouji
Harada (a), Feng Xu (b), Kyoichi Ono (b), Toshihiko Iijima (b)
and Akio Koizumia (a)
(a) Department of
Health and Environmental Sciences, Kyoto University Graduate
School of Medicine, Kyoto, Japan
(b) Department of Pharmacology, Akita University School of Medicine,
Akita, Japan
Perfluorooctane sulfonate
(PFOS) and perfluorooctanoate (PFOA) are amphiphiles found ubiquitously
in the environment, including wildlife and humans, and are known
to have toxic effects on physiological functions of various
tissues. We investigated the effects of PFOS and PFOA on action
potentials and L-type Ca2+ currents, ICaL, in isolated guinea-pig
ventricular myocytes using whole-cell patch-clamp recording.
In current-clamp experiments, PFOS significantly
decreased the rate of spike, action potential duration, and
peak potential at doses over 10 µM.
In voltage-clamp experiments, PFOS increased
the voltage-activated peak amplitude of ICaL, and shifted
the half-activation and inactivation voltages of ICaL to hyperpolarization.
PFOA had similar effects PFOS, but showed significantly lower
potency. These findings are consistent with previous observations
for anionic n-alkyl surfactants, suggesting
that PFOS and PFOA may change membrane surface potential, thereby
eliciting general effects on calcium channels. These
findings provide further insights into the mechanisms of PFOA
and PFOS toxicities.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15667078
Environ Sci Technol.
2005 Jan 1;39(1):80-4.
Temporal trends of PFOS and PFOA in guillemot eggs from
the Baltic Sea, 1968--2003.
Holmstrom
KE, Jarnberg U, Bignert A.
Institute of Applied
Environmental Research (ITM), Stockholm University, SE-106 91
Stockholm, Sweden. katrin.holmstrom@itm.su.se
Perfluorooctane sulfonic
acid (PFOS) and perfluorooctanoic acid (PFOA) have recently
been identified as ubiquitous environmental contaminants. Although
they have been produced for 50 years, little is known about
when they first appeared in the environment and how their concentrations
have changed over time, particularly in response to the phase-out
of PFOS, which began in 2000. In this study temporal trends
in the concentrations of PFOS and PFOA in the Baltic Sea marine
environmentwere measured using archived
guillemot eggs. Samples collected
from Stora Karlso (Sweden) between 1968 and 2003 were
received from an environmental specimen bank and concentrations
of PFOS and PFOA were analyzed using HPLC coupled to ESI-MS/MS.
PFOA was not detected in any of the samples
(LOD 3 ng/g), but there was an
almost 30-fold increase in PFOS concentrations in the guillemot
eggs during the time period, from 25 ng/g in 1968 to 614 ng/g
in 2003 (wet weight). Regression
analysis indicated a significant trend, increasing on average
between 7 and 11% per year. A sharp peak in PFOS concentrations
was observed in 1997 followed by decreasing levels up to 2002,
but this cannot be linked to the PFOS phase-out, which occurred
at the end of this period.
PMID: 15667078
[PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15694468
Chemosphere. 2005
Mar;58(11):1471-96.
Synthesis of environmentally relevant fluorinated surfactants-a
review.
Lehmler
HJ.
Department of Occupational
and Environmental Health, College of Public Health, University
of Iowa, 100 Oakdale Campus #124 IREH, Iowa City, IA 52242-5000,
USA.
In the past years
there has been a growing interest in fluorinated persistent
organic pollutants such as perfluorooctanesulfonic acid, perfluorooctanesulfonamides,
perfluorinated carboxylic acids and fluorotelomer alcohols.
Although these compounds have probably been present in the environment
for many decades, we are only now beginning to realize that
these environmental contaminants may have serious environmental
and health effects. This article gives
a state-of-the-art review of synthetic approaches that have
been employed for the synthesis of these environmentally relevant
fluorinated compounds. Perfluorooctanesulfonic
acid derivatives, in particular, pose a problem because only
a few perfluorooctanesulfonic acid derivatives are available
from commercial sources-a fact that limits the ability of researchers
worldwide to further study these compounds. Because of
the limited literature available, this article also describes
synthetic approaches for shorter chain homologues or perfluoroether
analogues that can potentially be applied for the synthesis
of perfluorooctanesulfonic acid derivatives. The preparation
of typical starting materials for the synthesis of perfluorooctanesulfonic
acid derivatives such as the perfluoroalkanesulfonyl fluorides
and chlorides will be discussed. Subsequently, their conversion
into relevant perfluoroalkane sulfonate salts (R(F)SO(3)M),
sulfonamides (R(F)SO(2)NH(2)), N-alkyl sulfonamides (R(F)SO(2)NHR,
R=alkyl), N,N-dialkyl sulfonamides (R(F)SO(2)NR(2), R=alkyl),
sulfonamidoethanol (R(F)SO(2)NRCH(2)CH(2)OH, R=-H, -CH(3) or
-C(2)H(5)) and sulfonamidoacetates (R(F)SO(2)NRCH(2)CO(2)H,
R=-H, -CH(3) or -C(2)H(5)) will be described. Many perfluorinated
carboxylic acids and fluorotelomer alcohols are available from
commercial sources. The review of the synthesis of these two
classes of fluorinated compounds includes a review of their
industrial synthesis and the synthesis of relevant degradation
products.
PMID: 15694468
[PubMed - in process]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15679355
Anal Chem. 2005
Feb 1;77(3):864-70.
Development of a Solid-Phase Extraction-HPLC/Single
Quadrupole MS Method for Quantification of Perfluorochemicals
in Whole Blood.
Karrman
A, van Bavel B, Jarnberg U, Hardell L, Lindstrom G.
Man-Technology-Environment
Research Centre, Orebro University, SE-701 82 Orebro, Sweden,
and Institute of Applied Environmental Research, Stockholm University,
SE-106 91 Stockholm, Sweden.
A method for the
determination of perfluorooctanesulfonate (PFOS)
and perfluorooctanoic acid (PFOA)
simultaneously with 10 closely related perfluorochemicals
(PFCs) in human whole blood was developed and validated. PFOS
and PFOA are used in various applications, for example, as surfactants
and plastic additives, and are subject to environmental and
health research due to their persistence. The main part of the
data on PFCs in human blood is from serum samples, analyzed
mainly by ion pair extraction followed by high-performance liquid
chromatography (HPLC) and negative electrospray (ESI) tandem
mass spectrometry (MS/MS). The analytical method developed here
is suitable for human whole blood and involves solid-phase extraction
(SPE) and HPLC negative electrospray single quadrupole mass
spectrometry (HPLC/ES-MS). A whole blood aliquot was treated
with formic acid and extracted on a octadecyl (C18) SPE column.
The PFCs were isolated with methanol, and quantification was
performed using single quadrupole mass spectrometry and perfluoroheptanoic
acid as internal standard. Validation was performed in the range
0.3-194 ng/mL with recovery between 64 and 112% and limit of
detection in the 0.1-0.5 ng/mL range for 11 of the 12 PFCs studied.
We applied this method to 20 whole blood samples collected in
1997-2000 from the Swedish population in the ages 24-72. Eleven
of the 12 PFCs were detected, and they were quantitatively and
qualitatively confirmed using triple quadrupole LC/MS/MS analysis.
PFOS, perfluorooctanesulfonamide, perfluorohexanesulfonate,
PFOA and perfluorononanoic acid were quantified in all samples.
In addition, perfluorohexanoic acid, perfluorodecanoic acid,
perfluorodecanesulfonate, perfluoroundecanoic acid, perfluorododecanoic
acid, and perfluorotetradecanoic acid were detected in some
samples. This study shows that SPE and single quadrupole MS
can be applied for extraction and quantification of PFCs in
human whole blood, resulting in selectivity and low detection
limits.
PMID: 15679355
[PubMed - in process]
From
Science Direct
Environmental Toxicology
and Pharmacology - Volume 19, Issue 1 , January 2005,
Pages 57-70
Identification of genes responsive to PFOS using gene
expression profiling
Wenyue
Hu, Paul D. Jones, Trine Celius and John P. Giesy
Department of Zoology,
National Food Safety and Toxicology Center and Institute of
Environmental Toxicology, Michigan State University, East Lansing,
224 National Food Safety and Toxicology Center, MI 48824-1311,
USA
Perfluorooctane sulfonic acid (PFOS) is widely distributed in
the environment including in the tissues of wildlife and humans,
however, its mechanism of action remains unclear. Here, the
Affymetrix rat genome U34A genechip was used to identify alterations
in gene expression due to PFOS exposure. Rat hepatoma cells
were treated with PFOS at 2–50 mg/L (4–100 ?M)
for 96 h. Sprague-Dawley rats were orally dosed with PFOS
at 5 mg/kg/day for 3 days or 3 weeks. Genes that were significantly
(P <0.0025) induced were primarily genes for fatty acid metabolizing
enzymes, cytochrome P450s, or genes involved in hormone regulation.
Consistent expression profiles were obtained for replicate exposures,
for short-term and long-term in vivo exposures, and for acute
and chronic exposures. One major pathway
affected by PFOS was peroxisomal fatty acid B-oxidation,
which could be explained by the structural similarity between
PFOS and endogenous fatty acids.