|
Return to
Index
Page
Adverse Effects
ACTIVITY: Insecticide,
Adjuvant (unclassified)
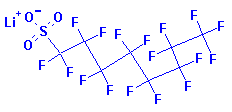
Systematic Names:
1-Octanesulfonic acid, 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluoro-,
lithium salt
Lithium heptadecafluorooctanesulphonate
Structure:
Reports
available from
The National Technical Information Service
(NTIS)
Order from NTIS by: phone at 1-800-553-NTIS (U.S. customers);
(703)605-6000 (other countries); fax at (703)605-6900; and
email at orders@ntis.gov. NTIS is located at 5285 Port Royal
Road, Springfield, VA, 22161, USA. |
| Report
No. |
Title |
Keywords |
CAS
Nos. |
NTIS/OTS0001378
EPA/OTS;
Doc #FYI-OTS-0500-1378 |
2000
- INFORMATION
ON PERFLUOROOCTANE SULFONATES: POST-1975 STUDIES PERTAINING
TO ENVIRONMENTAL EFFECTS, FATE & TRANSPORT, AND HEALTH EFFECTS,
W/ATTCHMNTS & CVR LTR DTD 05-04-00
|
3M
CO
PERFLUOROOCTANE SULFONATES
ENVIRONMENTAL FATE
PHYSICAL/CHEMICAL PROPERTIES
VAPOR PRESSURE
TRANSPORT PROCESSES
PHOTOLYSIS
BIODEGRADATION
BIOCONCENTRATION/BIOACCUMULATION
MONITORING
ENVIRONMENTAL EFFECTS
ACUTE TOXICITY
FISH-FRESHWATER
ALGAE
PLANT GROWTH OR DAMAGE TESTS
TISSUE CONCENTRATION
BIRDS
INVERTEBRATES
MOLLUSKS
MICROBIAL FUNCTION TESTS
BACTERIA
CHRONIC TOXICITY
HEALTH EFFECTS
GENOTOXICITY
DNA EFFECTS
MAMMALS
RATS
IN VITRO
GENE MUTATIONS
SUBCHRONIC TOXICITY
MONKEYS
ORAL
GAVAGE
COMBINED CHRONIC TOXICITY/CARCINOGENICITY
DIET
RABBITS
DERMAL
PHARMACO KINETICS
PARENTERAL
INTRAVENOUS
HUMANS |
2795-39-3
27619-97-2
29457-72-5
67584-42-3
70225-14-8 |
NTIS/OTS0001378
EPA/OTS;
Doc #FYI-OTS-1200-1378 |
2000
- DRAFT INITIAL ASSESSMENT REPORT:
PERFLUOROOCTANE SULFONIC ACID AND ITS SALTS, SUMMARIES OF
ALL HLTH & ENVIRNMNTL EXPOSR & RISK STUDIES AVAILABLE AS
OF 07-20-00, W/CVR LTR DTD 12-19-00 |
3M
CO
PERFLUOROOCTANE SULFONIC ACID AND ITS SALTS
HEALTH EFFECTS
GENOTOXICITY
GENE MUTATIONS
MAMMALS
MICE
IN VITRO
ORAL
GAVAGE
RATS
DNA EFFECTS
CHRONIC TOXICITY
COMBINED CHRONIC TOXICITY/CARCINOGENICITY
DIET
PHARMACO KINETICS
BIOCHEMISTRY
REPRODUCTION/FERTILITY EFFECTS
TERATOGENICITY
RABBITS
COMBINED TERATOGENICITY/REPRODUCTIVE EFFECTS
SUBCHRONIC TOXICITY
MONKEYS
ENVIRONMENTAL FATE
MONITORING
ENVIRONMENTAL EFFECTS
ACUTE TOXICITY
FISH-FRESHWATER
INVERTEBRATES
MOLLUSKS
CRITICAL LIFE STAGE TEST
PRIMARY EYE IRRITATION
DERMAL
INHALATION
PRIMARY DERMAL IRRITATION
EPIDEMIOLOGY
BACTERIA
CHROMOSOMAL EFFECTS
HUMANS
PARENTERAL
INTRAVENOUS |
1691-99-2
1763-23-1
2795-39-3
3825-26-1
24448-09-7
29081-56-9
29457-72-5
67584-42-3
70225-14-8
251099-16-8 |
NTIS/OTS0537870
EPA/OTS;
Doc #89-930000042S |
1992
- SUPPORT:
LETTER FROM 3M CO TO USEPA REGARDING STUDIES WITH LITHIUM
PERFLUOROOCTANE SULFONATE WITH COVER LETTER DATED 12-29-92
(SANITIZED)
HAZELTON
LABORATORIES |
3M
CO
LITHIUM PERFLUOROOCTANE SULFONATE
HEALTH EFFECTS
SUBCHRONIC TOXICITY
MAMMALS
RATS
ORAL
GAVAGE
DIET
REPRODUCTION/FERTILITY EFFECTS
TERATOGENICITY
RABBITS CAS |
29457-72-5 |
NTIS/OTS0537870
EPA/OTS;
Doc #88-930000106S |
1992
- INITIAL
SUBMISSION: LETTER FROM 3M CO TO USEPA REGARDING STUDIES
WITH LITHIUM PERFLUOROOCTANE SULFONATE IN THE RAT WITH
ATTACHMENTS AND COVER LETTER DATED 11-30-92
(SANITIZED)
BUSHY RUN RES CTR |
3M
CO
LITHIUM PERFLUOROOCTANE SULFONATE
HEALTH EFFECTS
ACUTE TOXICITY MAMMALS
RATS
ORAL
GAVAGE
INHALATION |
29457-72-5
|
Toxicologist
1994 Mar;14(1):162
Developmental
toxicity study with lithium perfluorooctane sulfonate in rats.
Henwood
SM, Costello AC, Osimitz TG
Lithium
Perfluorooctane sulfonate (LPOS) was administered by gavage
at 3, 6, or 12 mg/kg to mated Crl:CD„BR VAF/Plus„ female rats
once daily on days 6 through 15 of gestation. Body weights and
clinical observations were on days 0, 6, 9, 12, 16, 20 of gestation.
Food consumption was also measured. Cesarean sections were done
on surviving animals on day 20 of gestation, and the fetuses
were removed for examination. The dams were necropsied following
sacrifice. Clear maternal toxicity was observed in both the
6 and 12 mg/kg groups. Five out of 25 females in the 12 mg/kg
group did not survive to scheduled sacrifice. Both the 6 and
12 mg/kg groups had test material-related changes including
lower mean body weights, body weight gains, and food consumption.
Treatment at 12 mg/kg resulted in embryolethality
as evidenced by lower uterine weights, fewer live fetuses per
litter, reduced fetal bodyweights and lower percent of live
fetuses than the control treated. There was also significant
increased incidences of cleft palate (79%), and edema (36%).
Variations at this dose included reduced ossification of bone
and unossified bone. The no-observable-effect level (NOEL)
for LPOS for teratogenicity in rats is 6 mg/kg, whereas the
NOEL for maternal toxicity in rats is 3 mg/kg.
Teratology
1994 May;49(5):398
Developmental
toxicity study with lithium perfluorooctane sulfonate in rats.
Henwood
SM, McKee-Pesik P, Costello AC, Osimitz TG,
Hazleton
Wisconsin, Madison, WI.
Lithium
perfluorooctane sulfonate (LPOS) was administered by gavage
at 3, 6, or 12 mg/kg to mated Crl:CD„BR VAF/Plus„ female rats
once daily on Days 6 through 15 of gestation. Body weights and
clinical observations were done on Days 0, 6, 9, 16, and 20
of gestation. Food consumption was also measured. Cesarean sections
were done on surviving animals on Day 20 of gestation, and the
fetuses were removed for examination. The dams were necropsied
following sacrifice. Clear maternal toxicity was observed in
both the 6- and 12-mg/kg groups. Five of 25 females in the 12-mg/kg
group did not survive to scheduled sacrifice. Both the 6- and
12-mg/kg groups had material-related changes including
lower mean body weights, body weight gains, and food consumption.
Treatment at 12 mg/kg resulted in embryolethality as evidenced
by lower uterine weights, fewer live fetuses per litter, reduced
fetal bodyweights and lower percent of live fetuses than the
control groups. There was also
significant increases in the incidences of cleft palate (79%)
and edema (36%). Variations at this level included reduced ossification
of bone and unossified bone. The
no-observable-effect level (NOEL) of LPOS for teratogenicity
in rats is 6 mg/kg, whereas the NOEL for maternal toxicity in
rats is 3 mg/kg.
Teratology
1994 May;49(5):398
Developmental
toxicity study with lithium perfluorooctane sulfonate in rabbits.
Henwood
SM, McKee-Pesik P. Costello AC. Osimitz TG.
Hazleton
Wisconsin, Madison, WI.
Lithium
perfluorooctane sulfonate (LPOS) was administered by gavage
at 1, 2, or 4 mg/kg to mated New Zealand White female rabbits
once daily on Days 7 through 19 of gestation. Body weights and
clinical observations were made on Days 0, 7, 10, 13, 16, 20,
24, and 29 of gestation. Cesarean sections were done on surviving
animals on Day 29 of gestation, and the fetuses were removed
for examination. The does were necropsied following sacrifice.
Treatment at the 4-mg/kg level resulted in abortions; premature
deliveries; and lower mean body weights, lower body weight gains,
and lower gravid uterine weights than those of the control groups.
Cesarean sections revealed a treatment-related increase in postimplantation
losses at the 4-mg/kg level. Mean fetal body weight (mean =
10.01 g, n = 11) at the 4-mg/kg level was lower than that of
controls (mean = 39.93 g, n = 16) and represents developmental
toxicity. Fetal morphological examinations disclosed no evidence
of teratogenicity of LPOS in rabbits at any level. There were
increased incidences of unossified skeletal
structures at the 2- and 4-mg/kg levels. These variations
along with the lower fetal body weights suggest retarded development
at the 4-mg/kg level. The no-observable-effect level of LPOS
for developmental toxicity in rabbits is considered to be 2
mg/kg.
Return
to Lithium perfluorooctane sulfonate Index Page
|