|
Return to Index
Page
ACTIVITY:
Fungicide (azole)
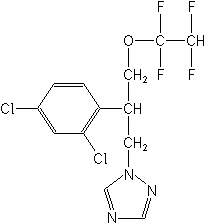
CAS Name:
1-[2-(2,4-dichlorophenyl)-3-(1,1,2,2-tetrafluoroethoxy)propyl]-1H-1,2,4-triazole
Structure:
| |
| Published
date |
Docket
Identification Number |
Details |
| April 11, 2007 |
EPA-HQ-OPP-2006-0576 |
Sipcam Agro USA,
Inc. and Isagro S.p.A. Pesticide
Tolerance. FINAL RULE.
• Human
Health Aggregate Risk Assessment for Triazole-derivative
Fungicide Compounds (1,2,4-Triazole, Triazole Alanine,
Triazole Acetic Acid). US EPA, February 7, 2006.
Hazard Characterization
(Page 13)
1,2,4-triazole (free triazole) is a metabolite common to
a number of triazole-derivative pesticides, and is found
in both mammalian (rat) and plant metabolism studies. Although
for most pesticides, mammals convert only a small proportion
to free triazole (less than 25%), two compounds
(tetraconazole and flusilazole) demonstrate relatively high
conversion (68-77%) in rat metabolism studies.
As a plant metabolite, and given the wide use of triazole-derivative
pesticides (used as fungicides on many crops as well as
on turf) free triazole is found in a variety of food commodities,
including animal byproducts. 1,2,4-triazole appears to be
relatively stable in the environment, and may be found in
rotational crops as well as in water. ...
Documents in the Federal Register
Docket:
Tetraconazole:
Human-Health Risk Assessment for Proposed Uses on Soybean,
Sugar Beet, Peanut, Pecan, and Turf.
January 26, 2007 - 105 pages
Docket #: EPA-HQ-OPP-2006-0576-0010
Tetraconazole.
Dietary Exposure and Risk Assessment. Application of Tetraconazole
to Pecan, Sugar Beet, and Soybean.
January
23, 2007 - 39 pages
Docket #: EPA-HQ-OPP-2006-0576-0011
Notice
of Filing Sipcam Agro USA (PP#9F6023 and PP#6F7084)
Undated - 9 pages
Docket #: EPA-HQ-OPP-2006-0576-0004
Notice
of Filing Sipcam Agro USA (PP#9F5066)
- Undated - 6
pages
Docket #: EPA-HQ-OPP-2006-0576-0005
Notice
of Filing PP#576971 Isagro -
Undated - 6 pages
Docket #: EPA-HQ-OPP-2006-0576-0007
Projected
Percent Crop Treated for the Fungicide Tetraconazole
- November 29,
2006 - 9 pages
Docket #: EPA-HQ-OPP-2006-0576-0009
| Commodity |
PPM |
| Aspirated grain fractions |
1.0 |
| Beet sugar, dried pulp |
0.15 |
| Beet sugar, molasses |
0.15 |
| Beet sugar, root |
0.05 |
| Cattle, fat |
0.02 |
| Cattle, liver |
0.20 |
| Cattle, meat |
0.01 |
| Cattle, meat byproducts (except liver) |
0.01 |
| Eggs |
0.02 |
| Goat, fat |
0.02 |
| Goat, liver |
0.20 |
| Goat, meat |
0.01 |
| Goat, meat byproducts (except liver) |
0.01 |
| Hog, fat |
0.01 |
| Hog, liver |
0.05 |
| Hog, meat |
0.01 |
| Hog, meat byproducts (except liver) |
0.01 |
| Horse, fat. |
0.02 |
| Horse, liver |
0.20 |
| Horse, meat |
0.01 |
| Horse, meat byproducts (except liver) |
0.01 |
| Milk |
0.01 |
| Milk, fat |
0.25 |
| Peanut |
0.03 |
| Peanut, oil |
0.10 |
| Pecan |
0.04 |
| Poultry, fat |
0.05 |
| Poultry, meat |
0.01 |
| Poultry meat byproducts |
0.01 |
| Sheep, fat |
0.02 |
| Sheep, liver |
0.20 |
| Sheep, meat |
0.01 |
| Sheep, meat byproducts (except liver) |
0.01 |
| Soybean, refined oil |
0.80 |
| Soybean, seed |
0.15 |
|
| December 20, 2006 |
EPA-HQ-OPP-2006-0576 |
Sipcam Agro
USA, Inc. Pesticide
petitions.
Pesticide Petition PP 6F7084.
in or on the food commodity
-- pecan at 0.05 ppm.
Pesticide Petition PP 9F6023.
in or on the food commodity
-- peanut, nutmeat at 0.05 ppm
-- peanut, refined oil at 0.15 ppm.
Pesticide Petition PP 9F5066.
in or on the food commodities
-- sugarbeet roots at 0.05 ppm
-- sugarbeet top at 3.0
-- sugarbeet dried pulp at 0.15 ppm
-- sugarbeet molasses at 0.15 ppm
-- meat of cattle, goat, horse and sheep at 0.05 ppm
-- liver of cattle, goat, horse and sheep at 4.0 ppm
-- fat of cattle, goat, horse and sheep at 0.30 ppm
-- meat byproducts, except liver, of cattle, goat, horse and
sheep at 0.10 ppm
-- milk at 0.05 ppm. |
| July 26, 2006 |
EPA-HQ-OPP-2006-0576 |
Isagro S.p.A.
Pesticide
petition: PP 5F6971.
To establish a new tolerance for residues of the fungicide tetraconazole
in or on food commodities
-- soybean, aspirated grain fractions/refined oil at 0.5 parts
ppm
-- soybean, seed at 0.1 ppm
-- poultry, fat at 0.05 ppm
-- poultry, egg/ liver/meat/meat byproducts at 0.01 ppm.
|
| Dec
21, 2005 |
EPA-HQ-OPP-2004-0388 |
Pesticide
Product Registrations; Conditional Approval.
This notice
announces Agency approval of applications submitted by Sipcam
Agro, USA Inc. to conditionally register the pesticide products,
Tetraconazole Technical, EPA Registration Number 60063-11
and Eminent 125 SL Fungicide, EPA Registration Number 60063-12,
containing a new active ingredient not included in any previously
registered products pursuant to the provisions of section
3(c)(7)(C) of the Federal Insecticide, Fungicide, and Rodenticide
Act (FIFRA), as amended.
EPA issued
a notice, published in the Federal
Register of October 20, 1999 which announced that Sipcam
Agro, USA Inc., 70 Mansell Court, Suite 230, Roswell, GA 30076
submitted an application to register the following two pesticide
products:
1. Tetraconazole Technical, (EPA File Symbol:
60063-RR). Active ingredient: Tetraconazole: at 97.0%. The
application for the product Tetraconazole Technical was approved
for manufacturing or formulating purposes on April 14, 2005,
to use for formulation into end-use products for
use on sugar beets (EPA Registration Number 60063-11).
2. Eminent 125SL Fungicide, (EPA File Symbol:
60063-RE). Active ingredient: Tetraconazole: at 11.6%. The
application for the product Eminent 125SL was approved on
April 14, 2005 for the control of Cercospora leaf spot and
powdery mildew disease of sugar beets
(EPA Registration Number 60063-12).
The Agency
has considered the available data on the risks associated
with the proposed use of tetraconazole, and information on
social, economic, and environmental benefits to be derived
from such use. Specifically, the Agency has considered the
nature and its pattern of use, application methods and rates,
and level and extent of potential exposure. Based on these
reviews, the Agency was able to make basic health and safety
determinations which show that use of tetraconazole during
the period of conditional registration will not cause any
unreasonable adverse effect on the environment, and
that use of the pesticide is, in the public interest.
A
paper copy of the fact sheet, which provides more detail on
this registration, may be obtained from the National Technical
Information Service (NTIS), 5285 Port Royal Rd., Springfield,
VA 22161. [See
EPA's April 2005 Fact Sheet - note that tetraconazole
is classified as "likely to be carcinogenic to humans“
based on the occurrence of liver tumors in male and female
mice.] In accordance with section 3(c)(2) of FIFRA,
a copy of the approved label, the list of data references,
the data and other scientific information used to support
registration, except for material specifically protected by
section 10 of FIFRA, are also available for public inspection.
Requests for data must be made in accordance with the provisions
of the Freedom of Information Act and must be addressed to
the Freedom of Information Office (A-101), 1200 Pennsylvania
Ave., NW., Washington, DC 20460-0001. The request should:
Identify the product name and registration number and specify
the data or information desired.
••
Comments
submitted to US EPA on this Notice from FAN Pesticide Project.
••
FOIA
Request for documents on tetraconazole. |
| August
31, 2005 |
OPP-2005-0223 |
Pesticide
Emergency Exemptions:
•
New Jersey. EPA authorized the use of
tetraconazole on soybeans to
control soybean rust; April 25, 2005 to November 10, 2007.
Contact: (Andrew Ertman)
• Tennessee. EPA authorized
the use of tetraconazole on soybeans
to control soybean
rust; April 25, 2005 to November 10, 2007. Contact: (Andrew
Ertman).
• Vermont. EPA authorized
the use of tetraconazole on soybeans
to control soybean
rust; June 23, 2005 to November 10, 2007. Contact: (Andrew
Ertman) |
| July
13, 2005 |
OPP-2005-0188 |
Pesticide
Emergency Exemptions:
Minnesota: Crisis: EPA authorized
the use of tetraconazole on soybeans
to control soybean rust; March 2, 2005, to March 1, 2008.
South Dakota: Quarantine: EPA authorized
the use of tetraconazole on soybeans
to control soybean rust; March 2, 2005, to March 1, 2008. |
| June
1, 2005 |
OPP-2005-0078 |
Pesticide
Tolerances for Emergency Exemptions. Final Rule.
This regulation establishes time-limited
tolerances for residues of tetraconazole in or on
| Commodity |
Parts
per million |
Expiration/
revocation date |
| Egg |
0.03 |
12/31/09 |
| Poultry,
fat |
0.004 |
12/31/09 |
| Poultry,
liver |
0.03 |
12/31/09 |
| Poultry,
meat |
0.0003 |
12/31/09 |
| Poultry,
meat byproduct, except liver |
0.002 |
12/31/09 |
| Soybean,
seed |
0.05 |
12/31/09 |
The
States of Minnesota and South Dakota, as lead state agencies
in what is essentially a ``national'' section 18 request for
all soybean growing States... Soybean
rust has been designated as a biosecurity threat and
therefore, it is important that control measures be available
for the disease.
For
purposes of this section 18 petition, parent tetraconazole
is being considered. The Agency does have concern about potential
toxicity of 1,2,4-triazole and two conjugates, triazolylalanine
and triazolyl acetic acid. These three compounds are metabolites
to most of the triazole-containing fungicides. To
support the extension of existing parent triazole-derivative
fungicide tolerances, EPA conducted an interim human health
assessment for aggregate exposure to 1,2,4-triazole. The exposure
and risk estimates presented in this assessment are overestimates
of actual likely exposures and therefore, should be considered
to be highly conservative. Based on this assessment EPA concluded
that for all exposure durations and population subgroups,
aggregate exposures to 1,2,4-triazole are not expected to
exceed its level of concern. This
assessment should be considered interim due to the ongoing
series of studies being conducted by the U.S.
Triazole Task Force (USTTF). Those
studies are designed to provide the Agency with more complete
toxicological and residue information for free triazole and
are expected to be submitted to the Agency in late 2004.
Upon
completion of the review of these data, EPA will prepare a
more sophisticated assessment based on the revised toxicological
and exposure databases.
The most recent estimated aggregate
risks resulting from the use of tetraconazole, are discussed
in the Federal Register of April 22, 2005 final rule (see
below) establishing tolerances
for residues of tetraconazole in/on sugarbeet and livestock
commodities. In
that prior action, risk was estimated assuming tolerance level
residues in all commodities for established and proposed tolerances,
including the tolerances for soybean and animal commodities
discussed in this document. |
| April
22, 2005 |
OPP-2004-0388 |
Sipcam
Agro USA.
7-year time-limited pesticide tolerance approved. Final
Rule.
Objections and requests for hearings
must be received on or before June 21, 2005.
Sipcam
Agro USA, Inc. requested these tolerances
under the Federal Food, Drug, and Cosmetic Act (FFDCA), as
amended by the Food Quality Protection Act of 1996 (FQPA).
Registrations will be limited to the
following States: Colorado, Minnesota,
Michigan, Montana, North Dakota, Nebraska, and Wyoming where
use has previously occurred under section 18 of FIFRA.
|
The following tolerances will expire
on November 30, 2012.
|
| Commodity |
Parts
per million date |
| Beet,
sugar, dried pulp |
0.15 |
| Beet,
sugar, molasses |
0.15 |
| Beet,
sugar, roots |
0.05 |
| Beet,
sugar, tops |
3.0
|
| Cattle,
fat |
0.30 |
| Cattle,
liver |
4.0 |
| Cattle,
meat |
0.05 |
| Cattle,
meat byproducts, except liver |
0.10 |
| Goat,
fat |
0.30 |
| Goat,
liver |
4.0 |
| Goat,
meat |
0.05 |
| Goat,
meat byproducts, except liver |
0.10 |
| Horse,
fat |
0.30 |
| Horse,
liver |
4.0 |
| Horse,
meat |
0.05 |
| Horse,
meat byproducts, except liver |
0.10 |
| Milk |
0.05 |
| Sheep,
fat |
0.30 |
| Sheep,
liver |
4.0 |
| Sheep,
meat |
0.05 |
| Sheep,
meat byproducts, except liver |
0.10 |
Background
and Statutory Findings. In
the Federal
Register of October 14, 1999, EPA issued a notice announcing
the filing of three pesticide petitions
(9F5066, 9F6023 and 7E4830) by Sipcam Agro, USA, Inc.,
300 Colonial Center Parkway, Roswell, GA 30076, formerly of
70 Mansell Court, Suite 230, Rosewell, GA 30076. The
petitions requested that 40 CFR part 180 be amended by establishing
tolerances for residues of the fungicide tetraconazole, in
or on the following raw agricultural commodities: beets, sugar
at 0.01 ppm, beets, sugar, roots at 0.1 ppm, beets, sugar,
tops at 7.0 ppm, beets, sugar, pulp, dried at 0.3 ppm, and
beets, sugar, molasses at 0.3 ppm, cattle, meat at 0.01 ppm,
cattle meat byproducts at 2.0 ppm, cattle fat at 0.1 ppm,
and milk at 0.02 ppm (9F5066);
peanuts meat (hulls removed) at 0.03 ppm, peanuts meal at
0.03 ppm, and peanuts oil at 0.1 ppm (9F6023); and
imported bananas at 0.2 ppm (7E4830). Petition 7E4830
was later withdrawn. Petition 9F6023 was placed in abeyance
by the petitioner. There were no comments received in response
to the notice of filing. The tolerances will expire on February
28, 2009.
Cancer
(oral, dermal, inhalation):
"Likely to be carcinogenic to humans" -
Q1
* = 2.30 x 10-2, based on male mouse
liver benign and/or malignant combined tumor rates
Aggregate cancer risk for U.S. population. The
estimated cancer risk for the proposed use on sugarbeets and
existing section 18 exemptions for soybeans is 2.5 x 10-6,
a value that falls within the Agency's risk standard for cancer
in the range of 1 x 10-6.
EPA
concluded that the additional safety factor for the protections
of infants and children could be removed:
Acute
dietary, females (13- 50 years of age):
Oral developmental toxicity study - rat. Developmental NOAEL
= 22.5 mg/kg/day, based on increased
incidence of small fetuses, and supernumerary ribs.
Chronic
dietary, all populations:
Chronic oral toxicity - dog. Systemic toxicity LOAEL = 2.95/3.33
(M/F) mg/ kg/day, based on absolute and relative kidney
weights and histopathological changes in the male
kidney
Metabolite
1,2,4-triazole:
The
Agency does have concern about potential toxicity to 1,2,4-triazole
and two conjugates, triazolylalanine and triazolyl acetic
acid, metabolites common to most of the triazole fungicides.
To support the extension of existing parent triazole-derivative
fungicide tolerances, EPA conducted
an interim human health assessment for aggregate exposure
to 1,2,4-triazole. Based on this assessment EPA concluded
that for all exposure durations and population subgroups,
aggregate exposures to 1,2,4-triazole are not expected to
exceed its level of concern. This
assessment should be considered interim due to the ongoing
series of studies being conducted by the U.S. Triazole Task
Force (USTTF). The toxicological
database for 1,2,4-triazole is incomplete. Preliminary
summary data presented by the USTTF to EPA indicate that the
most conservative endpoint currently available for use in
a risk assessment for 1,2,4-triazole is
a LOAEL of 15 mg/kg/day, based on body weight decreases in
male rats in the reproductive toxicity study (currently underway).
This endpoint, with an uncertainty factor of 1,000 was used
for both acute and chronic dietary risk, resulting
in an RfD of 0.015 mg/kg/day. The uncertainty factor
of 1,000 includes an additional 10X safety factor for the
protection of infants and children. The resulting PAD is 0.015
mg/kg/day.
Drinking
water.
-- EPA notes that ground water monitoring studies in New Jersey
and California showed maximum residues of 16.7 and 0.46 ppb,
respectively, which exceed the SCI-GROW estimates significantly.
-- With
the exception of the acute DWLOCs for infants and children
1 to 6 years old, all DWLOCs are greater than the largest
EEC (surface water estimate from use on turf). The
EEC's for these two population groups exceed the DWLOC's by
1.1 to 3.2-fold, a result typically interpreted to mean that
aggregate exposure exceeds EPA's level of concern.
The
Agency is planning to conduct a more sophisticated human health
assessment in 2005 following submission and review of the
ongoing toxicology and residue chemistry studies for 1,2,4-triazole.
Conditions:
The following conditions will be applied to the registration
of tetraconazole for use on sugarbeets:
1. Registration and tolerances
will be time-limited to allow review of triazole data and
completion of the triazole risk assessment.
2. Registrations will be limited
to the following States: Colorado, Minnesota, Michigan, Montana,
North Dakota, Nebraska, and Wyoming where use has previously
occurred under section 18 of FIFRA.
3. The registrant will be
required to provide one additional side-by-side sugarbeet
field trial comparing two and six applications of
Eminent 125SL at 0.10 lb ai/acre/application.
4. The registrant will be
required to provide a 28 day inhalation study.
5. Well documented estimates
of how many pounds of tetraconazole will be placed on the
market to treat sugarbeets.
6.
Tetraconazole use reporting on sugarbeets. This information
should be reported as how many pounds of tetraconazole will
be applied per acre on sugarbeets.
|
| Nov
10, 2004 |
OPP-2004-0232 |
Two
Pesticide Emergency Exemptions.
• Michigan
-
EPA authorized the use of tetraconazole
on sugar beets to control Cercospora
leafspot; May 4, 2004 to September 30, 2004. Contact: (Stacey
Groce).
• Wyoming - EPA authorized
the use of tetraconazole on sugar
beets to control Cercospora leafspot; May 4, 2004 to
September 30, 2004. Contact: (Stacey Groce) |
| May
5, 2004 |
OPP-2004-0116 |
Pesticide
Emergency Exemptions.
Colorado - EPA
authorized the use of tetraconazole on sugarbeet
to control Cercospora leaf spot; March 11, 2004 to September
30, 2004.
Minnesota - Specific: EPA authorized
the use of tetraconazole on sugarbeet
to control Cercospora leaf spot; March 11, 2004 to September
30, 2004.
Montana - Specific: EPA authorized
the use of tetraconazole on sugarbeet
to control Cercospora leaf spot; March 11, 2004 to September
30, 2004.
North Dakota - EPA authorized the
use of tetraconazole on sugarbeet
to control Cercospora leaf spot; March 11, 2004 to September
30, 2004. |
| April
28, 2004 |
OPP-2004-0102 |
Application
from Minnesota for Emergency Exemption.
EPA
has received a quarantine exemption request from the Minnesota
Department of Agriculture to use the pesticide tetraconazole
to treat up to 3.5 million acres of
soybeans to control soybean rust. The Applicant proposes
the use of a new chemical which has not been registered by
EPA.
-- The Applicant proposes to make no more than
two applications of
Eminent 125SL that may be made
by ground or air at a rate of 1.6 ounces
active ingredient/acre (13 fluid ounces of product per acre),
to 3.5 million acres of soybeans during the growing season
in Minnesota and
1.4 million gallons of product may be used.
-- This notice does not constitute a decision by EPA on the
application itself. The regulations governing section 18 of
FIFRA require publication of a notice of receipt of an application
for a specific exemption proposing use of a new chemical (i.e.,
an active ingredient) which has not been registered by EPA.
|
| Nov
26, 2003 |
OPP-2003-0358 |
7
Pesticide Emergency Exemptions. A ``specific exemption''
authorizes use of a pesticide against specific pests on a limited
acreage in a particular State. Most emergency exemptions are
specific exemptions.
-- Colorado Department of Agriculture.
Specific. EPA authorized the use of tetraconazole on sugarbeets
to control cercospora; April 28, 2003 to September 30, 2003.
-- Michigan Department of Agriculture.
EPA authorized the use of tetraconazole
on sugarbeets to control
cercospora; June 6, 2003 to September 30, 2003.
-- Minnesota Department of Agriculture.
Specific. EPA authorized the use of tetraconazole
on sugarbeets to control
cercospora; April 28, 2003 to September 30, 2003.
-- Montana Department of Agriculture.
Specific. EPA authorized the use of tetraconazole on sugarbeets
to control cercospora; April 28, 2003 to September 30, 2003.
-- Nebraska Department of Agriculture.
Specific. EPA authorized the use of tetraconazole on sugarbeets
to control cercospora; April 28, 2003 to September 30, 2003.
-- North Dakota Department of Agriculture.
EPA authorized the use of tetraconazole
on sugarbeets to control
cercospora; April 28, 2003 to September 30, 2003.
-- Wyoming Department of Agriculture.
EPA authorized the use of tetraconazole on sugarbeets
to control cercospora; April 28, 2003 to September 30, 2003. |
| June
25, 2003 |
OPP-2003-0179 |
Extension
of tolerances for emergency exemptions. FINAL RULE.
EPA has authorized under FIFRA section 18 the use of tetraconazole
on sugar beets for control of cercospora leaf spot in Colorado,
Montana, Nebraska, and Wyoming. This regulation extends
time-limited tolerances for residues of the fungicide tetraconazole.in
or on sugarbeets, and sugarbeet- related commodities, and for
secondary residues of triazole on animal commodities from livestock
fed sugarbeet by-products] at
-- 0.10 ppm in/on sugarbeet,
-- 6.0 ppm in/on sugarbeet top,
-- 0.20 ppm in/on sugarbeet dried pulp,
-- 0.30 ppm in/on sugarbeet molasses,
-- 0.050 ppm in milk,
-- 0.030 ppm in cattle, meat and meat
byproducts except kidney and liver,
-- 0.20 ppm in kidney,
-- 6.0 ppm in liver,
-- 0.60 ppm in fat
for an additional 2-year period.
These tolerances will expire and are revoked on December 31,
2005. The time-limited tolerances were originally published
in the Federal Register of December 6, 1999 (64 FR 68046) (FRL-6384-1).
|
| Aug
7, 2002 |
OPP-2002-0164 |
Emergency
Exemptions for pesticide use.
- Colorado
Department of Agriculture - EPA authorized the use of tetraconazole
on sugarbeet to control Cercospora;
May 29, 2002 to September 30, 2002.
- Michigan
Department of Agriculture - EPA authorized the use of tetraconazole
on sugarbeet to control Cercospora;
May 29, 2002 to September 30, 2002.
- Nebraska
Department of Agriculture - EPA authorized the use of tetraconazole
on sugarbeet to control Cercospora;
May 29, 2002 to September 30, 2002.
|
| Feb
6, 2002 |
OPP-181084 |
Receipt
of Application for Emergency Exemption, Solicitation of Public
Comment.
EPA has received specific exemption requests from the Minnesota
and North Dakota Departments of Agriculture to use the pesticide
tetraconazole to treat up to 1,660,000
acres of sugar beets to control Cercospora leaf spot.
The Applicants propose the use of a new chemical which has not
been registered by the EPA.
The Applicants propose to make no more than three applications
of tetraconazole, formulated as a liquid with 1 pound active
ingredient (a.i.) per gallon at a rate of 1.625 ounces a.i.
per acre, on up to 1,660,000 acres of sugar beets in North Dakota
and Minnesota. Use at this rate on the maximum number of acres
could result in application of a total of 168,594 pounds a.i.,
or 168,594 gallons of formulation. The proposed use season
is June 15 through September 30, 2002. |
| Nov
14, 2001 |
OPP-181082 |
- Pesticide
Emergency Exemptions. EPA authorized the use in:
- Colorado:
on sugar beets to control cercospora leafspot (Cercospora
Beticola); June 15, 2001 to September 30, 2001.
- Minnesota:
on sugar beets to control cercospora leafspot; June 15,
2001 to Sept 30, 2001.
- Montana:
on sugar beets to control cercospora leafspot (Cercospora
Beticola); June 15, 2001 to Sept 30, 2001.
- Nebraska:
on sugar beets to control cercospora leafspot (Cercospora
Beticola); July 1, 2001 to Sept 30, 2001.
- North
Dakota: on sugar beets to control cercospora leafspot; June
15, 2001 to Sept 30, 2001.
- Wyoming:
on sugar beets to control cercospora leafspot (Cercospora
Beticola); June 15, 2001 to Sept 30, 2001.
|
| July
19, 2001 |
OPP-301146 |
Extension
of Tolerances for Emergency Exemptions. - FINAL RULE.
EPA has authorized the use of tetraconazole on sugarbeets for
control of cercospora leafspot in Michigan, Montana, Colorado,
Nebraska, and Wyoming. This regulation extends time-limited
tolerances for residues of the fungicide tetraconazole in or
on sugarbeets, and sugarbeet-related commodities, and for secondary
residues of triazole on animal commodities from livestock fed
sugarbeet by-products at 0.10 ppm on sugarbeet, 6.0 ppm in sugarbeet
top, 0.20 ppm in sugarbeet dried pulp, 0.30 ppm in sugarbeet
molasses, 0.050 ppm in milk, 0.030 ppm in cattle, meat and meat
byproducts except kidney and liver, 0.20 ppm in kidney, 6.0
ppm in liver, and 0.60 ppm in fat for an additional 2- years.
These tolerances will expire and are revoked on December 31,
2003. |
| Dec
6, 1999 |
OPP-300931 |
Pesticide
Tolerances for Emergency Exemptions. - FINAL RULE.
This regulation establishes time-limited tolerances for residues
of tetraconazole in or on sugar beets, and sugar beet-related
commodities, and for secondary residues of triazole on animal
commodities from livestock fed sugar beet by-products. This
action is in response to EPA's granting of an emergency exemption
under provisions of section 18 of the Federal Insecticide, Fungicide,
and Rodenticide Act, authorizing use of the pesticide on sugar
beets. CATTLE: liver at 6.0 ppm; fat at 0.60 ppm; kidney at
0.20 ppm; meat at 0.03 ppm; meat byproducts (except kidney and
liver) at 0.03 ppm. Milk at 0.05 ppm. BEET, SUGAR: dried pulp
at 0.20 ppm; molasses at 0.30 ppm; roots at 0.10 ppm; tops at
6.0 ppm. The tolerances will expire and will be revoked on December
31, 2001. |
| Oct
20, 1999 |
OPP-
30482 |
- SIPCAM
AGRO - Registration
Applications for TWO pesticide products.
- Tetraconazole
Technical. Fungicide. Active ingredient: Tetraconazole at
97.0%. Tetraconazole Technical is intended for the formulation
into end-use products for use on sugar beets, peanuts, and
turf. Type registration: Conditional.
- Eminent
125SL Fungicide. Active ingredient: Tetraconazole at 11.6%.
Eminent 125SL is intended for the control of Cercospora
leaf spot and powdery mildew disease of sugar beets; early
and late leaf spot, rust, web blotch, and southern blight
of peanuts and dollar spot, copper spot, rust, Southern
blight, brown patch, red thread, anthracnose, powdery mildew,
etc. diseases of turf. Type registration: Conditional.
|
| Oct
14, 1999 |
PF-893 |
- SIPCAM
AGRO - Three
Petitions for Pesticide Tolerances for residues of Tetraconazole
in or on the Raw Agricultural Commodities of:
- Petition
9F5066: beets, sugar at 0.01 ppm; beets, sugar, roots at
0.1 ppm; beets, sugar, tops at 7.0 ppm; beets, sugar, pulp,
dried at 0.3 ppm; and beets, sugar, molasses at 0.3 ppm;
and in animal commodities of milk at 0.02 ppm; cattle, meat
at 0.01 ppm; cattle meat byproducts at 2.0 ppm and cattle
fat at 0.1 ppm.
- Petition
9F6023: peanuts meat (hulls removed) at 0.03 ppm, peanuts
meal at 0.03 ppm, and peanuts oil at 0.1 ppm.
- Petition
7E4830: imported bananas at 0.2 ppm.
|
|