|
Return to
Index Page
Adverse Effects
Abstracts
ACTIVITY:
Herbicide (sulfonylurea)
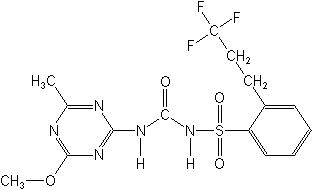
CAS Name:
N-[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]-2-(3,3,3-trifluoropropyl)benzenesulfonamide
Structure:
| |
| Date
Published |
Docket
Identification Number |
Details |
| Dec
31, 2002 |
OPP-2002-0343 |
SYNGENTA
-
Pesticide Tolerance
Petition; for residues in or on the raw agricultural commodities
| Commodity |
PPM |
| cereal
grains group (except rice and wild rice) grain |
0.10
|
| cereal
grains group (except rice and wild rice) fodder |
0.10
|
| cereal
grains group (except rice and wild rice) straw |
0.02 |
| cereal
grains group (except rice and wild rice) hay |
0.20
|
| milk |
0.01
|
| meat,
fat, kidney, liver, and meat by-products of cattle, goats,
hogs, horses, and sheep |
0.05
|
--
Acute toxicity. EPA has set an acute reference dose of 0.1
milligram/kilogram/day (mg/kg/day) based upon a no observed
adverse effect level (NOAEL) of 10 mg/kg/day from the rat
acute neurotoxicity study (lowest observed adverse
effect level (LOAEL) of 250 mg/kg/day due to reduced motor
activity and body temperature in males and impaired righting
reflex in females) and a 100-fold uncertainty factor (UF).
-- In a rat teratology study, evidence of maternal toxicity
(decreased body weight gain and
reduced food consumption) and developmental toxicity (increased
incidence of skeletal variations that was not significantly
different from the historical control) was found at the maximum
tolerated dose of 400 mg/kg.
-- Chronic toxicity. In the 1-year dog
chronic dosing study, the NOAEL was 1.84 mg/kg/day
based on hematologic and clinical
chemistry effects and incidence of lipofuscin
accumulation in the liver at 18.6 mg/kg/day.
-- In the 18-month mouse carcinogenicity study, there was
no evidence of carcinogenic effects up to the highest dose
tested (HDT) of 1,062 mg/kg/day. The NOAEL was 1.71 mg/kg/day
in males, and 100 mg/kg/ day in females based on increased
incidence/severity of centrilobular
hepatocellular hypertrophy.
-- A 2-year chronic feeding/carcinogenicity study in rats
indicated systemic NOAEL of 7.9 mg/kg/day was based on decreased
body weight and body weight gain, hematopoietic
effects (males), and possibly increased serum GGT and
decreased liver, kidney, and adrenal
weights (females) at 79.9 mg/kg/ day. There was uncertain
evidence of carcinogenicity with slight increases in the incidence
of mammary gland adenocarcinomas in
females at 95.7 and 205.8 mg/kg/day, slight increase
in incidence of benign testicular interstitial
cell tumors at 79.9 and 160.9 mg/kg/day (significant
trend only). Considering the weight of the evidence,
the EPA Reference Dose Committee previously concluded that
the chemical should be classified as a
Group D carcinogen (inadequate evidence), not classifiable
as to human carcinogenicity. The HIARC (meeting December 2,
1999) accepted the previous conclusions and updated
the cancer classification to the new classification: ``data
are inadeqate,'' with no new studies required. EPA
has set a chronic reference dose of 0.02 mg/kg based on a
NOAEL of 1.84 mg/kg in a dog feeding study and a 100-fold
UF. |
| Aug
7, 2002 |
OPP-2002-0122 |
Cancellation
of Pesticides for Non-payment of Year 2002 Registration Maintenance
Fees:
Table 2.--Section 3 Registrations Canceled for Non-Payment
of Maintenance Fee:
Prosulfuron + Atrazine Herbicide
- EPA Registration number 000100-00956
|
| Dec
22, 1999 |
PF-907 |
NOVARTIS
- Pesticide
Tolerance Petition; for residues of prosulfuron in or
on the raw agricultural commodities cereal grains group (except
rice and wild rice) grain at 0.01 ppm; cereal grains group
(except rice and wild rice) forage at 0.10 ppm; cereal grains
group (except rice and wild rice) fodder at 0.01 ppm; cereal
grains group (except rice and wild rice) straw at 0.02 ppm;
cereal grains group (except rice and wild rice) hay at 0.20
ppm; milk at 0.01 ppm; and meat, fat, kidney, liver and meat
by-products of cattle, goats, hogs, horses, and sheep at 0.05
ppm. |
| Aug
25, 1999 |
PF-882 |
NOVARTIS
- Petition
to extend time-limited tolerances for residues of prosulfuron,
in or on the cereal grains group (except rice and wild rice)
grain at 0.01 ppm; cereal grains group (except rice and wild
rice) forage at 0.10 ppm; cereal grains group (except rice
and wild rice) fodder at 0.01 ppm; cereal grains group (except
rice and wild rice) straw at 0.02 ppm; cereal grains group
(except rice and wild rice) hay at 0.20 ppm; milk at 0.01
ppm; and meat, fat, kidney, liver and meat byproducts of cattle,
goats, hogs, horses, and sheep at 0.05 ppm until December
31, 2001. |
| Augt
4, 1997 |
OPP-300523 |
Pesticides
Subject to Tolerance Reassessment. |
| May
29, 1996 |
OPP-300416A/R2243 |
CIBA-GEIGY
- Extension
of Pesticide Tolerance. - FINAL RULE. This rule
establishes tolerances for residues of the herbicide in or
on the raw agricultural commodities corn (forage, fodder,
grain and fresh [including sweet kernels plus cobs with husks
removed]), milk, and meat, fat and meat by-products, of cattle,
goats, hogs, horses, and sheep. The regulation establishes
the maximum permissible level for residues of the herbicide
in or on corn. These tolerances will expire on December 31,
1999. |
| May
29, 1996 |
PP
5F4469/R2225 |
CIBA-GEIGY
- Time-Limited
Pesticide Tolerance. - FINAL RULE. US EPA is establishing
time-limited tolerances for residues of the herbicide in or
on the raw agricultural commodities cereal grains group (except
rice and wild rice), grain; cereal grains group (except rice
and wild rice), forage; cereal grains group (except rice and
wild rice), fodder, cereal grains group (except rice and wild
rice), straw; and cereal grains group (except rice and wild
rice), hay. These tolerances will expire on December 31, 1999.
|
| April
17, 1996 |
P
5F4469/ P650 |
Proposed
Pesticide Tolerance. EPA proposes to establish time-limited
tolerances for residues of the herbicide in or on the raw agricultural
commodities cereal grains group (except rice and wild rice),
grain at 0.01 ppm; cereal grains group (except rice and wild
rice), forage at 0.10 ppm; cereal grains group (except rice
and wild rice), fodder at 0.01 ppm, cereal grains group (except
rice and wild rice), straw at 0.02 ppm; and cereal grains group
(except rice and wild rice), hay at 0.20 part per million (ppm).
Tolerances expire on December 31, 1999. |
| March
6, 1996 |
OPP-300416 |
CIBA-GEIGY
- Petition
for Proposed Pesticide Tolerance. EPA proposes to extend
the tolerances for residues of the herbicide in or on the
raw agricultural commodities corn (forage, fodder, grain and
fresh [including sweet kernels plus cobs with husks removed])
at 0.01 ppm, milk at 0.01 ppm, and fat, kidney, liver, meat
by-products, of cattle, goats, hogs, horses, and sheep at
at 0.05 ppm. The Agency has not completed the regulatory assessment
of our science findings; therefore, the Agency is proposing
to extend these tolerances until December 1999. |
| Aug
17, 1995 |
PF-629 |
CIBA-GEIGY
- Correction
of May 24, 1995 Federal Register. Notice of filing of
petition by American Cyanamid Co. for the herbicide prosulfuron
is corrected to state that the filing is by Ciba-Geigy Corp.,
not American Cyanamid Co. The notice appeared in the Federal
Register of May 24, 1995 |
| July
26, 1995 |
na |
CIBA-GEIGY
- Approval
of Pesticide Product Registrations. This notice announces
Agency approval of applications submitted by Ciba-Geigy Corporation,
to conditionally register the pesticide products Peak; (formerly
Exceed WG) for weed control in field corn (grown for grain,
silage, or seed), popcorn, and sweet corn (EPA Registration
Number 100-763). Exceed, for weed control in field corn (grown
for grain, silage, or seed), and popcorn (EPA Registration
Number 100-774). Prosulfuron Technical; for formulation into
herbicides for the use on corn (EPA Registration Number 100-762). |
| May
24, 1995 |
PF-625;
FRL 4951-3 |
AMERICAN
CYANAMID - Petition
for Pesticide Tolerance for combined residues on cereal grains,
cereal grains forage, fodder, and straw (except rice and wild
rice) at 0.02 ppm. |
| May
10, 1995 |
PP
4F4336/R2133 |
CIBA-GEIGY
- Pesticide
Tolerances. - FINAL RULE. This rule establishes
time-limited tolerances, to expire on December 31, 1995, for
residues in or on the raw agricultural commodities corn (fodder,
forage, grain and fresh [including sweet kernels plus cobs
with husks removed]) at 0.01 ppm, milk at 0.01 part per million
(ppm), and fat, kidney, liver, meat, and meat byproducts of
cattle, goats, hogs, horses, and sheep at 0.05 ppm. |
| Nov
2, 1994 |
na |
CIBA-GEIGY
- Tolerance
Petition Filing for residues of the new herbicide prosulfuron
in or on the following raw agricultural commodities: corn,
forage at 0.02 ppm; corn, fodder at 0.02 ppm; corn, grain
at 0.02 ppm; corn, fresh (including sweet kernels plus cobs
with husks removed) at 0.02 ppm; milk at 0.02 ppm; meat, fat
and meat byproducts, kidney and liver of cattle, goats, hogs,
horses, and sheep at 0.10 ppm; poultry, fat, kidney, liver,
meat and meat byproducts at 0.10 ppm; eggs at 0.10 ppm. |
| Aug
18, 1994 |
OPP-30371 |
CIBA-GEIGY
- Product
Applications for: Exceed WG Herbicide; for postemergence
weed control in field corn (grown for grain, silage, or seed),
popcorn, and sweet corn. Prosulfuron Technical; for formulation
into herbicides. |
|