|
Return to
Abstracts
Fomesafen Adverse Effects
Fomesafen sodium Adverse Effects
ACTIVITY:
Herbicide
(Diphenyl
ether)
CAS Name
for Fomesafen:
5-[2-chloro-4-(trifluoromethyl)phenoxy]-N-(methylsulfonyl)-2-nitrobenzamide
Structure
for Fomesafen:
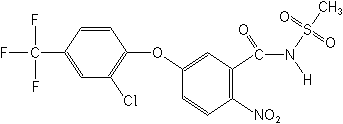
| Fomesafen
Adverse Effects:
Blood
Body
Weight Decrease
Bone
Cancer: Possible Human Carcinogen -
LIVER
Liver
Stomach |
Fomesafen, sodium
Adverse
Effects:
Cancer:
Possible Human Carcinogen - LIVER
|
| Fomesafen
Environmental Effects:
Phototoxic
Pesticide.
Potential
ground water contaminant |
Fomesafen, sodium
Environmental Effects:
As
of Dec 2003: No data available
|
Regulatory
Information
(only comprehensive for the US) |
| Fomesafen |
Fomesafen, sodium
|
| US
EPA Registered: |
Yes |
Yes |
| US
EPA PC Code: |
123802 |
123802 |
| California
Chemical Code |
- |
5086 |
| US
Tolerances: |
CFR
180.433 |
CFR
180.433 |
US
EPA Permit Date
and Registrant: |
1987,
ICI |
- |
| European
Commission: |
Not
allowed to be used as an active ingredient after July 25,
2003, with the following exceptions:
France
- Soybean, bean
Italy - Soybean, bean, pea
UK - Pea, bean, lupin
. |
-- |
Registered
use in
(includes only a limited list of countries)
|
Canada,
Hungary, South Africa, Tanzania, UK, US
Canada:
Dry beans, Lima beans, Snap beans, Soybeans
|
US |
US
Maximum Residue Levels
|
In
2003, the US Code of Federal Regulations (CFR) listed both
Fomesafen and the Sodium salt of fomesafen with a tolerance
of 0.05 ppm on Soybean.
The
2004 CFR did not list Fomesafen, only the sodium salt.
|
US:
Soybean
Over
the years EPA has granted several Emergency Exemptions for
the use of "Fomesafen." |
| Other
Information |
| Molecular
Formula: |
C15H10Cl
F3 N2O6S |
C15H10ClF3N2O6S
¥Na |
| Entry
Year: |
1977 |
- |
| Inventing
Company: |
Zeneca |
- |
| Manufacturers: |
Syngenta |
Zeneca,
BASF |
| Other
Names: |
Flexstar
Reflex
Tornado
Twister
Typhoon |
PP
021
Flexstar
FLEX
Reflex
Tornado
Twister
Typhoon
BAS 530 04 |
| Of
special interest: |
PAN
Data for
Fomesafen
PAN
Data
for Fomesafen, sodium
Also
Fomesafen, sodium: PAN Bad Actor
for Acute toxicity |
| EPA
released the following documents on March 28, 2007 (for
more documents, see the Federal Register entry below): |
Fomesafen Summary Document.
(45 pages)
EPA-HQ-OPP-2006-0239-0003
|
Fomesafen: HED Registration Review
Problem Formulation Document (February 28, 2007).
This document describes
the scope of work necessary to support the registration
review. (8 pages)
EPA-HQ-OPP-2006-0239-0004
|
Human
Health Documents for Registration Review
|
Fomesafen: HED Registration Review
Problem Formulation Document (February 28, 2007).
This document describes
the scope of work necessary to support the registration
review. (8 pages)
EPA-HQ-OPP-2006-0239-0004
|
Fomesafen Sodium: Human Health Risk
Assessment for a Proposal to Amend Use on Soybeans,
and Proposals to Add Uses on Cotton, Dry Bean, and
Snap Bean, PC Code: 123802, Petition Nos: 1E6228,
9F50568, 6E4653, DP Barcode D325797 (February 28,
2006). This document describes
all the human health risks from the use of fomesafen.
(59 pages)
EPA-HQ-OPP-2006-0239-0005
|
6 EFED Problem Formulation for Fomesafen
Registration Review (Transmittal Memo) (February 13,
2007). This is the transmittal
memo for the ecological risk problem formulation.
(1 page)
EPA-HQ-OPP-2006-0239-0006
|
Registration Review – Ecological
Risk Assessment Problem Formulation for Fomesafen
This document describes
the ecological risk problem formulation.
(25 pages) EPA-HQ-OPP-2006-0239-0007
|
Ecological Risk Assessment for New
Uses of Fomesafen on Cotton (DP 302766), Snap beans
(DP 314014), and Dry Beans (DP 314112) (January 30,
2007) This is the transmittal
memo for the Ecological Risk Assessment. (4
pages) EPA-HQ-OPP-2006-0239-0008
|
Ecological Risk Assessment for Use
of Fomesafen on Cotton (DP302766), Snap Beans (DP314014)
and Dry Beans (DP314112) (January 2006). This
document describes the toxicity of fomesafen to animals
and plants in the environment. (55
pages) EPA-HQ-OPP-2006-0239-0009
|
|
| Material
Safety Data Sheets & Labels |
2005
- Schedule for Reregistration & Tolerance Reassessment (RED)
is expected to be November 2005. Contact at EPA: Stephanie Plummer
(703) 305-0076; plummer.stephanie@epa.gov . According to EPA:
Through the pesticide reregistration and tolerance reassessment
programs, EPA is assessing risks and making risk management
decisions for older pesticides. These decisions are summarized
in documents known as REDs, IREDs, and TREDs. By making decisions
according to the schedule below, EPA will meet its statutory
deadlines for completing reregistration and tolerance reassessment.
Some of the decision dates presented in the schedule may change
due to the dynamic nature of the review process. Any pesticide
decisions that are not completed during the current fiscal year
will be rescheduled for the following year. EPA is committed
to meeting its reregistration and tolerance reassessment deadlines.
http://www.epa.gov/pesticides/reregistration/decision_schedule.htm
|
| Fomesafen.
November
26, 2002 - European Commission: one of 320 pesticides to be
withdrawn in July 2003.
However, specific exemptions for
continued use of Fomesafen have been allowed on Soybean, bean
in France; Soybean, bean, pea in Italy; and Pea,
bean, lupin in the U.K. "Some
320 substances used in plant protection products (PPPs) Ð including
insecticides, fungicides and herbicides Ð are to be withdrawn
from the market by 25 July 2003 as part of the European Commission's
new approach to the evaluation of active substances in plant
protection products. This aims to improve safeguards to ensure
that all such products in use are safe for the environment and
human health. Users, wholesalers and retailers of plant protection
products will need to be aware of whether the products they
use or sell are likely to be withdrawn, so as to prevent them
being left with stocks of unusable material. Those concerned
should contact their national authority to check the authorisation
status for any particular product. The Regulation (n¡ 2076/2002
of 20 November 2002), with the list of the 320 substances, has
now been published in the Offical Journal. Ref: MIDDAY EXPRESS.
News from the Press and Communication Service's midday briefing.
|
| TOXNET
profile from Hazardous Substances Data Bank |
| Novemer
21, 2001 -
Fomesafen,
Schedule No. 1288. Canada
Food and Drug Regulations: Maximum
Residue Limit of 0.05 ppm in Lima beans; approved by Canada's
Pest Management Regulatory Agency (PMRA), of Health Canada |
| March
7, 2001 -
Phototoxic Pesticides:
US EPA Memo Requesting Phototoxicity Study
Protocol for Light-Dependent Peroxidizing Herbicides |
May
24, 2000 - Cancer
Assessment Document. Evaluation
of the Carcinogenic Potential of Diclofop-Methyl. (Second
Review). Final Report. Cancer Assessment Review Committee,
Health Effects Division, US EPA Office of Pesticide Programs.
"There are eight diphenyl ethers that are structurally
similar to diclofop-methyl. Of the chemicals, fomesafen
sodium, haloxyfop-methyl
(Verdict), oxyfluorfen,
acifluorfen sodium, nitrofen,
and lactofen were
reviewed in the initial CPRC report. All of these chemicals
induced liver adenomas and carcinomas in rats and/or mice..."
Organofluorine
pesticides highlighted in red |
| US
Map of Pesticide Use: 1992-1995 |
| October
2001-
Fomesafen sodium.
Glossary of Pesticide Chemicals.
A listing of pesticides subject to analysis
of residues in foods and feeds by the US Food and Drug Administration. |
| Abstracts |
| Fomesafen
(CAS No. 72178-02-0).
2000
Toxic Release Inventory: Brief Summary. |
Rationale
for US EPA to add Fomesafen (CAS No. 072178-02-0)
to the Toxic Release Inventory
Decreased
plasma cholesterol and triglycerides and increased liver
weights (reversible at 7 days post-treatment) were observed
at 50 mg/kg/day (only dose tested) when administered in
the diet of rats for 4 weeks. In a 90-day rat study, dietary
administration of 5 mg/kg/day (LOEL) produced alterations
in lipid metabolism and increases in liver weight. The NOEL
was 0.25 mg/kg/day. In a 26-week dog study, dietary administration
of 25 mg/kg/day (LOEL) produced alterations in lipid metabolism
and liver changes (changes not defined). The NOEL was 1
mg/ kg/day. Liver toxicity (increased liver masses, discolored
hepatocytes, and pigmented Kupffer cells) was observed in
a 2-year rat feeding study at 50 mg/kg/day (LOEL). The NOEL
was 5 mg/kg/day. Metabolism studies have shown that fomesafen
accumulates in the liver. EPA believes that there is sufficient
evidence for listing fomesafen on EPCRA section 313 pursuant
to EPCRA section 313(d)(2)(B) based on the available hepatic
toxicity data for this chemical.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals
from Federal Insecticide, Fungicide, Rodenticide Act (FIFRA)
Active Ingredients to EPCRA Section 313. U. S. Environmental
Protection Agency, Washington, DC (1993). As cited by US
EPA in:
Federal
Register: January 12, 1994. Part
IV. 40 CFR Part 372. Addition of Certain Chemicals; Toxic
Chemical Release Reporting; Community Right-to-Know; Proposed
Rule.
|
US
Federal Register
••
Note: Due to length, the following is a partial
list. Click here
to see full list of FR entries.
|
| Published
Date |
Docket
Identification Number |
Details |
September 12, 2007
|
EPA-HQ-OPP-2007-0097 |
Tolerance Actions. FINAL RULE. EPA is inceasing the following tolerances for fomesafen from 0.025 to 0.05 ppm in order to harmonize with the Canadian MRLs in support of North American Free Trade Agreement (NAFTA) (see June 2007 proposal):
Bean, dry - 0.05 ppm
Bean, snap, succulent - 0.05 ppm |
| June 6, 2007 |
EPA-HQ-OPP-2007-0097 |
Proposed
Tolerance Action. EPA is proposing to modify certain tolerances
for fomesafen.
-- Currently, the tolerance
in 40 CFR 180.433(a) for residues of fomesafen in/on bean,
dry and bean, snap, succulent are each 0.025 ppm (May 3, 2006
(71 FR 25945) (FRL-8062-6). The Canadian
MRL is 0.05 ppm bean, dry and bean, snap, succulent. EPA proposes
increasing the tolerances in 40 CFR 180.433(a) for residues
of fomesafen in/on bean, dry and bean, snap, succulent from
0.025 to 0.05 ppm in order to harmonize
with the Canadian MRLs in support of North American Free Trade
Agreement (NAFTA). The Agency determined that the increased
tolerances are safe; i.e., there is a reasonable certainty
that no harm will result from aggregate exposure to the pesticide
chemical residue.
-- A RED for fomesafen was not needed because
it was registered after November 1, 1984 and not subject to
reregistration eligibility, and its tolerances were reassessed
prior to completion of a TRED, such that a RED for fomesafen
was no longer needed because EPA made a safety finding which
reassessed its tolerances according to FQPA standards.
| Commodity |
Current Tolerance |
Proposed Tolerance |
| bean, dry |
0.025 ppm |
0.05 ppm |
| bean, snap, succulent |
0.025 ppm |
0.05 ppm |
|
| March
28, 2007 |
EPA-HQ-OPP-2006-0239 |
Pesticide
Registration Review; New Docket Opened for Review and Comment.
Pesticide Registration Review; New Docket Opened for Review
and Comment. As directed by FIFRA section 3(g), EPA is periodically
reviewing pesticide registrations to assure that they continue
to satisfy the FIFRA standard for registration--that is, they
can still be used without unreasonable adverse effects on
human health or the environment. The implementing regulations
establishing the procedures for registration review appear
at 40 CFR part 155. A pesticide's registration review begins
when the Agency establishes a docket for the pesticide's registration
review case and opens the docket for public review and comment.
At present, EPA is opening registration review for fomesafen.
Comments must be received on or before
June 26, 2007.
Fomesafen Summary Document. (45
pages)
EPA-HQ-OPP-2006-0239-0003 |
| Human Health
Documents for Registration Review |
| Fomesafen: HED Registration Review Problem
Formulation Document (February 28, 2007). This
document describes the scope of work necessary to support
the registration review. (8
pages) EPA-HQ-OPP-2006-0239-0004 |
Fomesafen Sodium: Human Health Risk
Assessment for a Proposal to Amend Use on Soybeans, and
Proposals to Add Uses on Cotton, Dry Bean, and Snap Bean,
PC Code: 123802, Petition Nos: 1E6228, 9F50568, 6E4653,
DP Barcode D325797 (February 28, 2006).
This document describes all
the human health risks from the use of fomesafen. (59
pages)
EPA-HQ-OPP-2006-0239-0005 |
6 EFED Problem Formulation for Fomesafen
Registration Review (Transmittal Memo) (February 13, 2007).
This is the transmittal memo
for the ecological risk problem formulation. (1
page)
EPA-HQ-OPP-2006-0239-0006 |
Registration Review – Ecological
Risk Assessment Problem Formulation for Fomesafen
This document describes the
ecological risk problem formulation. (25
pages)
EPA-HQ-OPP-2006-0239-0007 |
Ecological Risk Assessment for New Uses
of Fomesafen on Cotton (DP 302766), Snap beans (DP 314014),
and Dry Beans (DP 314112) (January 30, 2007)
This is the transmittal memo
for the Ecological Risk Assessment. (4
pages)
EPA-HQ-OPP-2006-0239-0008 |
Ecological Risk Assessment for Use of
Fomesafen on Cotton (DP302766), Snap Beans (DP314014)
and Dry Beans (DP314112) (January 2006).
This document describes the
toxicity of fomesafen to animals and plants in the environment.
(55 pages)
EPA-HQ-OPP-2006-0239-0009 |
| Appendices
for the EFED Ecological Risk Assessment (ERA) for new
uses of fomesafen on cotton, snap beans, dry beans |
Appendix A – Environmental Fate
Studies. (5 pages)
EPA-HQ-OPP-2006-0239-0010 |
Appendix B – PRZM EXAMS Output.
(11 pages)
EPA-HQ-OPP-2006-0239-0011 |
Appendix C - TREX Output, (2
pages)
EPA-HQ-OPP-2006-0239-0012 |
Appendix D - TerrPlant Output.
EPA-HQ-OPP-2006-0239-0013
Use docket
search at the Federal Register. See pieces of this
document here
and here |
Appendix E - Ecological Effects Data.
(12 pages)
EPA-HQ-OPP-2006-0239-0014 |
Appendix F - EIIS Output. (2
pages)
EPA-HQ-OPP-2006-0239-0015 |
Appendix G - RQ Method and LOCs. (2
pages)
EPA-HQ-OPP-2006-0239-0016 |
Appendix H-1 – Endangered and
Threatened Species. (26 pages)
EPA-HQ-OPP-2006-0239-0017 |
Appendix H-2 – ES Exclusion Chart.
(1 page)
EPA-HQ-OPP-2006-0239-0018 |
| Use and Usage
Information for Registration Review |
Fomesafen Screening-Level Usage Analysis
(SLUA) (November 24, 2006)
This document provides the available
estimates of pesticide usage data for fomesafen that is
used on agricultural crops in the United States. (4
pages)
EPA-HQ-OPP-2006-0239-0019 |
Appendix A (November 22, 2006)
This table list the food/feed
and non-food/non-feed uses eligible for reregistration.
(1
page)
EPA-HQ-OPP-2006-0239-0020 |
Tolerance Salt of Fomesafen Summary
Report
This document list the tolerances
established for fomesafen. (1
page)
EPA-HQ-OPP-2006-0239-0021 |
Fomesafen Registrations
This document lists the pending
and active registrations for fomesafen. (7
pages)
EPA-HQ-OPP-2006-0239-0022 |
| Incident Reports
for Fomesafen |
Review of Fomesafen Incident Reports
This document reports poisoning
incident data on the active ingredient Fomesafen. (4
pages)
EPA-HQ-OPP-2006-0239-0023
|
Ecological Incident Summary for Registration
Review Snapshot. (1 page)
EPA-HQ-OPP-2006-0239-0024 |
Incident Report (Part A) (November 15,
2006). (3 pages)
EPA-HQ-OPP-2006-0239-0025 |
|
| August 25, 2006 |
EPA-HQ-OPP-2006-0659 |
Pesticide
Emergency Exemptions; Agency Decisions and State and Federal
Agency Crisis Declarations
-- Fomesafen on snap or dry beans
in Arkansa, Delaware, Indiana, Maine, Maryland, Michigan, Minnesota,
Missouri, Oklahoma |
| June 7, 2006 |
EPA-HQ-OPP- 2006-0387 |
Pesticide
Emergency Exemptions; Agency Decisions and State and
Federal Agency Crisis Declarations.
-- New York: EPA authorized the use of fomesafen on dry
and snap beans to control broadleaf weeds; June 1,
2006 to August 30, 2006.
-- Pennsylvania: Specific: EPA authorized the use of fomesafen
on snap beans to control broadleaf
weeds; June 1, 2006 to August 30, 2006.
|
| May 3, 2006 |
EPA-HQ-OPP-2006-0073 |
IR-4 and Syngenta
Crop Protection. Pesticide
tolerance. FINAL
RULE.
-- Bean, dry at 0.025 ppm
-- Bean, snap, succulent at 0.025 ppm
-- Cotton, gin byproducts at 0.025 ppm
-- Cotton, undelinted seed at 0.025ppm
-- Soybean at 0.05 ppm |
| March
1, 2006 |
EPA-HQ-OPP-2006-0073
and
Pesticide
petition numbers (PP)
1E6228,
6E4653, and 9F5068 |
IR-4
& SYNGENTA.
New
Tolerance. PPs 1E6228, 6E4653, and 9F5068. Interregional
Research Project No. 4 (IR-4) and Syngenta Crop Protection,
Inc. proposes to establish tolerances for residues of the
herbicide sodium salt of fomesafen in or on food commodities
| PP# |
Commodity |
PPM |
| PP
1E6228 |
dry
beans |
0.025 |
| PP
6E4653 |
snap
beans |
|
9F5068 |
cotton
seed and cotton gin byproducts |
An analytical
method using chemical derivatization followed by gas chromatography
(GC) with Nitrogen-Phosphorus detection (NPD) has been developed
and validated for residues of fomesafen in snap/dry beans,
cotton seed and cotton gin byproducts, as well as for other
crops. After homogenization, the samples are extracted with
acidified acetonitrile. After addition of water and additional
acid, the extract was submitted to liquid/liquid partition.
The residue is transferred to dichloromethane followed by
acetone and derivatized with iodomethane in the presence of
anhydrous potassium carbonate. A silica gel column cleanup
is done with dichloromethane:hexane as the eluent. The final
extract is transferred to toluene and analyzed by GC/NPD.
The limit of quantitation is 0.025 ppm. |
|