|
Return to
Adverse Effects
Abstracts
MSDS & Labels
ACTIVITY: Herbicide
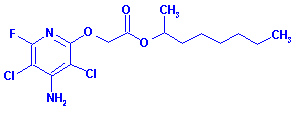
Systemic Name: 1-Methylheptyl
((4-amino-3,5-dichloro-6-fluoropyridin-2-yl)oxy)acetate
NOTES:
When Fluroxypyr
is normally used as a salt or an ester, the identity of which should
be stated, for example fluroxypyr-butoxypropyl
[154486-27-8], fluroxypyr-meptyl [81406-37-3].
Structure:
| Adverse
Effects:
Bone
Endocrine: Adrenal
Endocrine:
Testicular
Kidney |
| Environmental
Effects:
Highly
Toxic to Crustaceans and Zooplankton
Slightly
to Highly Toxic to Fish |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Yes |
| US
EPA PC Code: |
128968 |
| US
Tolerances: |
CFR
180.535 |
Registered
use in
(includes only a limited list of countries)
|
Australia,
Canada, Hungary, US |
US
Maximum Residue Levels permitted
in food commodities
|
Permitted
in or on 54 food commodities, including:
Aspirated grain fractions,
Barley (forage, grain, hay, straw),
Cattle (fat, kidney, meat, meat byproducts),
Corn (Field: forage, grain, stover; Sweet (forage, kernels,
stover),
Goat (fat, kidney, meat, meat byproducts),
Grass (forage and hay),
Hog (fat, kidney, mean, meat byproducts),
Horse (fat, kidney, meat, meat byproducts),
Milk, Oat (forage, grain, hay, straw), Onion,
Sheep (fat, kidney, meat, meat byproducts),
Wheat (forage, grain, hay, straw) |
| Other
Information |
| Molecular
Formula: |
C15-H21-Cl2-F-N2-O3
|
| Manufacturers: |
DowAgro |
| Other
Names: |
Starane
Starane + saber
Starane plus salvo
Starance plus sword.
Vista
DOWCO 433 MHE
XRM-5084
ACETIC
ACID, ((4-AMINO-3,5-DICHLORO-6-FLUORO-2-PYRIDINYL)OXY)-, 1-METHYLHEPTYL
ESTER
AMINO-3,5-DICHLORO-6-FLUORO-2-PYRIDINYL)OXY)ACETIC
ACID, 1-METHYLHEPTYL ESTER
METHYLHEPTYL
((4-AMINO-3,5-DICHLORO-6-FLUORO-2-PYRIDINYL)OXY)ACETATE
|
| Manufacture
site: |
ISRAEL:
Agan Chemical, Ashdod
FRANCE:
Dow Elanco, Drusenheim |
| Of
special interest: |
| PAN
Data |
| Material
Safety Data Sheets & Labels |
| See
also Fluroxypyr |
| Australia: Time-limited
permit from 10 November 2003 to 31 October 2005 for use
of the pesticide product Starane 200 Herbicide on Non-Crop Areas.
(Note: Victoria is not included in this permit because their
‘control-of-use’ legislation means that a permit
is not required to legalise this off-label use in VIC). |
| June
2002 - In Australia when Fluroxypyr is used as a "Herbicide
on poppies" no
maximum residue levels are required. Ref:
June 2002. Table 5. Uses of substances where maximum
residue limits are not necessary. Australian National Registration
Authority for Agricultural Veterinary Chemicals. The MRL Standard.
Maximum residue limits in food and animal feedstuff. http://www.nra.gov.au/residues/mrl5.pdf
|
| September
30, 1998 - Pesticide
Fact Sheet. US EPA |
| October
2001 - Glossary
of Pesticide Chemicals. A listing
of pesticides subject to analysis of residues in foods and feeds
by the US Food and Drug Administration. |
| Abstracts |
US
Federal Register
••
Note: Due to length, the following is a partial
list. Click here
to see full list of FR entries.
|
| Date
Published |
Docket
Identification Number |
Details |
| August 25, 2006 |
EPA-HQ-OPP-2006-0659 |
Pesticide
Emergency Exemptions; Agency Decisions and State and Federal
Agency Crisis Declarations
• Colorado: EPA authorized the use of fluroxypyr on onions
to control volunteer potatoes; June 6, 2006 to July 31, 2006. |
| April 21, 2006 |
EPA-HQ-OPP-2005-0536 |
IR-4 and Dow
AgroSciences. Pesticide
petition: PP 3E6775.
proposal to establish a tolerance for the combined residues
of the herbicide fluroxypyr MHE and its metabolite fluroxypyr
(expressed as total fluroxypyr) in or on food commodities
-- garlic and shallot (bulb) at 0.03 ppm
-- onion (dry bulb) at 0.03 ppm |
| Feb
10, 2005 |
OPP-2005-0025 |
Removal
of Expired Time-limited Tolerances for Emergency Exemptions.
FINAL
RULE.
• 10. Fluroxypyr 1-methylheptyl ester. Time-limited
tolerances for
cattle kidney, goat kidney, grass forage
and hay, hog kidney, horse
kidney, milk and sheep kidney are being removed from
Sec. 180.535
because they expired on December 31, 2004. |
| Jan
26, 2005 |
OPP-2005-0008 |
Pesticide
Tolerances for Emergency Exemptions. FINAL RULE.
EPA, on its own initiative, in accordance with sections 408(e)
and 408(l)(6) of the Federal Food, Drug, and Cosmetic Act
(FFDCA), 21 U.S.C. 346a, is establishing a tolerance for combined
residues of the herbicide fluroxypyr 1-methylheptyl ester
1-methylheptyl ((4-amino-3,5-dichloro-6-fluoro-2-pyridinyl)oxy)acetate
and its metabolite fluroxypyr
[((4-amino-3,5-dichloro-6-fluoro-2-pyridinyl)oxy)acetic acid],
in or on
This
tolerance will expire and is revoked on June 30, 2007.
Although this tolerance will expire and is revoked on June
30, 2007, under section 408(l)(5) of the FFDCA, residues of
the pesticide not in excess of the amounts specified in the
tolerance remaining in or on onion after that date will not
be unlawful, provided the pesticide is applied in a manner
that was lawful under FIFRA, and the residues do not exceed
a level that was authorized by this tolerance at the time
of that application. EPA will take action
to revoke this tolerance earlier if any experience with, scientific
data on, or other relevant information on this pesticide indicate
that the residues are not safe.
Because
this tolerance is being approved under emergency conditions
identified in the state of Colorado,
EPA has not made any decisions about whether fluroxypyr 1-methylheptyl
ester meets EPA's registration requirements for use on onion
or whether a permanent tolerance for this use would be appropriate.
|
| Jan
14, 2004 |
OPP-2003-0402 |
Extension
of Tolerances for Emergency Exemptions. FINAL
RULE. EPA has authorized under FIFRA
section 18 the use of fluroxypyr 1-methylheptyl ester on field
corn and sweet corn for control of volunteer potatoes in
Washington, Oregon, Idaho, and Wisconsin. This regulation
extends time-limited tolerances for residues of the herbicide
fluroxypyr 1-methylheptyl ester and
its metabolite fluroxypyr
in or on
| corn,
sweet, K + CWHR |
0.05
ppm |
| corn,
sweet, forage |
2.0
ppm |
| corn,
sweet, stover |
2.5
ppm |
| corn,
field, grain |
0.05
ppm |
| corn,
field, forage |
2.0
ppm |
|
corn, field, stover |
2.5 ppm |
for
an additional 3-year period. These
tolerances will expire and are revoked on December 31, 2006.
Time-limited tolerances were originally published in the Federal
Register of August 5, 1998 |
| Dec
31, 2003 |
OPP-2003-0377 |
Dow AgroSciences.
Pesticide tolerance.
FINAL RULE. Tolerances
are established for
combined residues of fluroxypyr 1-methylheptyl ester and its
metabolite Fluroxypyr [((4-amino-3,5-dichloro-6-fluoro-2-pyridinyl)oxy)
acetic acid], free and conjugated, all expressed as fluroxypyr.
-- 870.4300 Carcinogenicity--Rats NOAEL
= 100 mg/kg/day. LOAEL = 500 mg/kg/day based on chronic
progressive kidney glomerulonephropathy. no evidence
of carcinogenicity
--
870.3800 Reproduction and fertility
Parental/Systemic NOAEL = 100 mg/kg/day effects (Males) /
500 mg/kg/day (Females) LOAEL = 500 mg/kg/day (Males)
/ 1,000 mg/kg/ day (Females), based on
kidney effects (M&;F) and increased deaths (F).
| Commodity |
PPM |
| Cattle, kidney |
1.5 |
| Corn, field, forage |
1.0 |
| Corn, field,
grain |
0.02 |
| Corn, field, stover |
0.5 |
| Corn, sweet, forage |
1.0 |
| Corn, sweet, kernel plus
cob with husks removed |
0.02 |
| Corn, sweet, stover |
2.0 |
| Goat, kidney |
1.5 |
| Grass, forage |
120 |
| Grass, hay |
160 |
| Hog, kidney |
1.5 |
| Horse, kidney |
1.5 |
| Milk |
0.3 |
| Sheep, kidney |
1.5 |
| Sorghum, grain, forage |
2.0 |
| Sorghum, grain, grain |
0.02 |
| Sorghum, grain, stover |
4.0 |
Conditions:
i. Additional field trials - conduct
and submit four (4) additional field trials in Regions III
(1 trial), V(1 trial), XI(1 trial), and XII(1 trial). Residue
analysis of sweet corn field trial samples should
avoid using the DowElanco Method ACR 90.8, due to matrix
interference cited in PP#2G04066.
ii. Storage stability data - submit to support the sweet corn
field trial data.
iii. 28-Day Inhalation Toxicity Study |
••
Note: Due to length, the above is a partial list.
Click here
to see full list of FR entries.
|
|

