|
Return to Fluorouracil
Adverse Effects
ACTIVITY: Former
Insect Chemosterilant; now
used as a chemotherapeutic drug
Systematic
Name: 2,4(1H,3H)-Pyrimidinedione,
5-fluoro-
Structure:
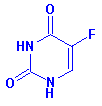
Rationale
for US EPA to add Fluorouracil to the Toxic Release Inventory
A major
use of fluorouracil is in the palliative treatment of carcinoma
of the colon, rectum, breast, stomach, and pancreas that
is not amenable to surgery or irradiation. The major toxic
effects of fluorouracil are on the normal, rapidly proliferating
tissues particularly of the bone marrow and lining of the
gastrointestinal tract. Leukopenia, predominantly of the
granulocytopenic type, thrombocytopenia, and anemia occur
commonly with intravenous fluorouracil therapy at doses
ranging from 6 to 12 mg/kg. Pancytopenia and agranulocytosis
also have occurred.
Developmental
abnormalities or other effects on newborns were reported
in offspring of women receiving 150 or 240 mg/kg fluorouracil
intravenously during weeks 11 to 14 or 20 to 31 of pregnancy.
In addition, maternal toxicity to the reproductive organs,
toxicity to the fetus, and developmental abnormalities have
been reported in mice, rats, and hamsters receiving oral,
intraperitoneal, or intramuscular doses of fluorouracil
ranging from 10 to 700 mg/kg.
Chronic
neurotoxic effects were noted in dogs fed fluorouracil at
a dietary dose of 2 mg/kg/day for 6 months. In this study,
animals were examined at the end of 3 months and 6 months.
At the end of the experiment, or at death, the brain was
removed and examined (only one dog survived the entire 6-month
period). Histological sections of the brain showed the presence
large multiple monolocular vacuoles in the wall of the fornix
of the third ventricle.
EPA
believes that there is sufficient evidence for listing fluorouracil
on EPCRA section 313 pursuant to EPCRA section 313(d)(2)(B)
based on the toxicity of this substance to bone marrow,
and on the developmental and chronic neurotoxicity data
for this chemical.
USEPA/OPPT.
Support Document for the Health and Ecological Toxicity
Review of TRI Expansion Chemicals. U. S. Environmental Protection
Agency, Washington, DC (1993). As
cited by US EPA in:
Federal
Register: January 12, 1994. Part
IV. 40 CFR Part 372. Addition of Certain Chemicals; Toxic
Chemical Release Reporting; Community Right-to-Know; Proposed
Rule.
|
Chemical
Sterilants, Virus Vectors, Insect Control
Author:
M.L. SHARMA
Acta Hort. (ISHS) 94:403-406, 1981
Abstract: Several
chemosterilants have been tested in the laboratory and chemicals
as 5-fluorouracil, thiotepa, tepa, metepa and apholate have
given excellent results in the reduction of reproductive
capacity as well as in sterility of Tetranychus urticae,
Aphis fabae, Acyrthosiphon pisum, Myzus persicae and Macrosiphum
euphorbiae. Because of their perennial nature, the fruit
trees provide excellent testing material and as such the
above mentioned chemosterilants may be tested in experimental
orchards against Aphis pommi, Myzus persicae and Tetranychus
species that abound these perennials.
Since the sterility by 5-fluorouracil and thiotepa seems
to be prolonged upto F2 generation, these products may have
a long range effect in the aphid control. Since M.
persicae and A. pommi are virus vectors, not only we can
expect an eradication of the virus vector but also an attenuation
of the viruses itself because of the nature of chemosterilants
used. In the use of thiotepa (maximum of 0.5%) the stability
of the chemosterilant can be prolonged when used in solutions
with sodium bicarbonate. These applications will probably
bring a solution to virus vector eradication as well as
to viruses propagation. At this stage, however, no guarantee
seems available against the destruction of useful insects
as Coccinellidae and Chrysopidae. The above suggestions
should be considered as experimentation hypothesis under
controlled and isolated orchards. In
all cases great care must be taken in the application of
chemosterilants because of the sterility and side effects
they may cause to the animal and human life.
|
TOXNET
profile from Hazardous Substances
Data Bank |
"readily
crosses the blood-brain barrier and distributes into CSF and
brain tissue." |
| "Potential
Adverse Effects on Fetus: Exposure in first trimester: skeletal
abnormalities; hypoplasia of aorta, lungs, thymus, and gastrointestinal
tract; and urinary tract abnormalities. Fetus exposed in third
trimester had cyanosis and clonus." |
| "the
incidence of malformations, particularly those affecting the
tail, hindlimb bud, and brain, was increased." |
| inhibits
"DNA, RNA, and protein synthesis" |
| PDR
entry for EFUDEX ¨ (ICN) fluorouracil) TOPICAL SOLUTIONS AND
CREAM Ref: Physicians Desk Reference
(On-line 2001) [Efudex® is made by by Hoffmann-La
Roche Inc.] |
"Fluorouracil
produced petite mutations in Saccharomyces cerevisiae and was
positive in the mironucleus test (bone marrow cells of male
mice)." |
| "There
is evidence that the metabolism of fluorouracil in the anabolic
pathway blocks the methylation reaction of deoxyuridylic acid
to thymidylic acid. In this manner fluorouracil interferes with
the synthesis of deoxyribonucleic acid (DNA) and to a lesser
extent inhibits the formation of ribonucleic acid (RNA). Since
DNA and RNA are essential for cell division and growth, the
effect of fluorouracil may be to create a thymine deficiency
which provokes unbalanced growth and death of the cell. The
effects of DNA and RNA deprivation are most marked on those
cells which grow more rapidly and take up fluorouracil at a
more rapid rate." |
| "cases
of miscarriage and a birth defect (ventricular septal defect)
have been reported when Efudex was applied to mucous membrane
areas during pregnancy. " |
|