|
Return to Ethalfluralin
Adverse Effects
ACTIVITY: Herbicide
(2,6-Dinitroaniline)
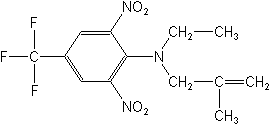
CAS NAME:
N-ethyl-N-(2-methyl-2-propenyl)-2,6-dinitro-4-(trifluoromethyl)benzenamine
Structure:
| Adverse
Effects:
Adverse
Effects:
Ataxia
Bladder
Blood
Body Weight Decrease
Bone (including Cleft Palate)
Cancer: Possible Human Carcinogen - BREAST,
BLADDER, KIDNEY
Cholesterol
Clastogenic /Mutgenic
Developmental toxicant
Endocrine: Breast
Endocrine: Ovary
Kidney
Liver
Teratogen
Environmental |
| Environmental
Effects:
Persists
in soil
Highly to very highly toxic to rainbow trout and bluegill
sunfish.
Highly toxic to marine/estuarine fish, mollusks, and shrimp
on an acute basis
Endangered species levels of concern are exceeded for freshwater
organisms and estuarine/marine invertebrates |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Yes |
| US
EPA PC Code: |
113101 |
| California
Chemical Code |
2166 |
| US
Tolerances: |
CFR
180.416 |
| FDA
LMS Code: |
721 |
US
EPA Permit Date
and Registrant: |
1983,
Elanco |
Registered
use in
(includes only a limited list of countries)
|
Canada,
Hungary, US |
US
Maximum Residue Levels permitted
in food commodities
|
US
- permitted in or on 26 food commodities,
including:
Bean
(dry, seed), Canola (seed), Peanut, Pea (dry, seed), Safflower
(seed), Soybean, Sunflower (seed), Cantaloupe,
Cucumber, Melon, Muskmelon, Watermelon |
| Other
Information |
| Molecular
Formula: |
C13H14
F3 N3O4 |
| Entry
Year: |
1973 |
| Inventing
Company: |
Dow |
| Manufacturers: |
|
| Other
Names: |
EL-161,
Sonalan |
| Of
special interest: |
| PAN
Data |
| Material
Safety Data Sheets & Labels |
| March
1995 - US
EPA's Registration Eligibility Decision (RED) |
| January
1995 -
US EPA's RED Factsheet
- (10 pages) |
| Abstracts
& NTIS studies |
| Map
of US pesticide use: 1992-1995 |
| Herbicide
products - partial list. |
| April
2000 -
Food and Drug Administration
Pesticide Residue Monitoring. Table
3. Pesticides detectable by methods used in 1999 regulatory
monitoring. |
| October
2001 - Glossary
of Pesticide Chemicals. A listing
of pesticides subject to analysis of residues in foods and feeds
by the US Food and Drug Administration. Also available at: http://vm.cfsan.fda.gov/~acrobat/pestglos.pdf |
| March
2000 - Cenex Supply and Marketing, Inc. (CSMI) in Quincy, Grant
County, Washington, operated a liquid fertilizer and soil fumigant
storage and distribution facility. Seious contamination resulted
from the landspreading of pesticide sediment sludges on agricultural
land. Among the many pesticides found in soil samples, three
herbicides (trifluralin, vernolate, and ethalfluralin), one
insecticide (chlorpyrifos), one pesticide (disulfoton), and
three metals (chromium, beryllium, and cadmium) exceeded one
or more health-based comparison values in CSMI soil. The population
of Quincy is 3,715. This report characterizes the community's
concerns about excess cancer and disease rates, very rare abnormalities
such as Rubinstein-Taybi
Syndrome, and ill health as not "unusual"
- a typical ATSDR-explantion handed out to very sick communities. |
US
Federal Register
••
Note: Not all entries are listed
below. Click here
to view all entries published in the FR.
|
| Date
Published |
Docket
Identification Number |
Details |
| August
31, 2005 |
OPP-2005-0195 |
Dow
AgroSciences & IR-4.
Pesticide Tolerance
Petition. Petitions: PP
1E6326, PP 2E6360 and PP 2E6466 for
the raw agricultural commodities:
| Commodity |
Part
Per Million |
| canola |
0.05
|
| crambe |
0.05
|
| mustard
seed |
0.05
|
| potato |
0.05 |
| rapeseed |
0.05
|
| dill |
0.05 |
•
The summary of the petition was prepared by Interregional
Research Project Number 4 (IR-4). IR-4 submitted the
petitions on behalf of the registrant, Dow
AgroSciences LLC, who prepared this notice of filing.
•
Genotoxicty.
Ethalfluralin was weakly mutagenic in activated strains TA1535
and TA100 of salmonella typhimurium, but not in strains TA1537,
TA1538, and TA98 in an Ames assay... In Chinese hamster ovary
cells, ethalfluralin was negative without S9 activation, but
it was clastogenic with activation.
• Reproductive and developmental
toxicity. The developmental NOAEL in rats was 1,000
mg/kg/day, the highest dose. In rabbits
the NOAELs for maternal and developmental toxicity were 75
mg/kg/day. The maternal LOAEL at 150 mg/kg/day was based on
abortions and decreased food consumption.
These effects as well as decreased
weight gain, enlarged liver, and orange urine were found at
300 mg/kg/day. In this study developmental toxicity was observed.
The developmental LOAEL in rabbits was
150 mg/kg/day, based on slightly increased resorptions,
abnormal cranial development, and increased sternal variants.
In a three-generation rat reproduction study, the parental
NOAEL was 12.5 mg/kg/day. The parental LOAEL was 37.5 mg/kg/day,
based on depressed mean body weight
gains in males in all generations. No treatment-related
effects were noted on reproductive parameters and the NOAEL
was 37.5 mg/kg/day or greater. A 7-month multigeneration bridging
study was conducted with doses equivalent to 0, 8, 20, or
61 mg/kg/day in the
diet of Fischer 344 rats. The parental NOAEL was 20 mg/kg/day.
The parental LOAEL was 61 mg/kg/day based on
increased liver weights.
•
Chronic toxicity.
Ethalfluralin was administered to Fisher
344 rats in the diet for 2 years in combined chronic toxicity
and carcinogenicity replicate studies.
The doses were equivalent to 0, 4.2, 10.7, or 32.3
mg/kg/day. The NOAEL for systemic effects was 32.3 mg/kg/day.
Mammary gland fibroadenomas were found in dosed female rats
at statistically significant incidences in the mid and high
doses. EPA's Office of Pesticide Program's
Carcinogenicity Peer Review Committee concluded that, ethalfluralin
should be classified as Group C, a possible human carcinogen,
based on increased mammary gland fibroadenomas and adenomas/fibroadenomas
combined in female rats. The tumor incidences were statistically
significant at both the mid and high dose, and exceeded the
upper range of historical controls.
Based on a low dose extrapolation, the Q1* of 8.9 x 10-2 (mg/kg/day)-1
has been calculated. Based on both registered and proposed
product uses, exposure to ethalfluralin from food is estimated
to not exceed a lifetime cancer risk of 8.47 x 10-7. Cancer
risks of less than 1 x 10-6 are generally considered to be
negligible.
• Beagle dogs were given 0, 4,
20, or 80 mg/kg/day orally, by capsule, for 1 year. The NOAEL
was 4 mg/kg/day. The LOAEL was 20 mg/kg/day, based on increased
urinary bilirubin, variations in erythrocyte morphology, increased
thrombocyte count, and increased erythroid series of the bone
marrow. Elevated alkaline phosphatase levels were found at
the two higher doses and siderosis of the liver at the high
dose. |
| ••
Note: Not all entries are listed
above. Click here
to view all entries published in the FR. |
|