|
Return
to Picoxystrobin Index Page
Activity:
Fungicide
(strobin)
Structure:
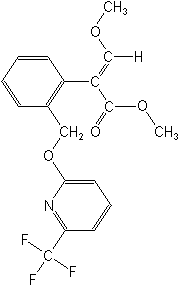
Adverse Effects:
Body
Weight Decrease
Bone
Endocrine: Testicular
Toxicologically
significant compounds:
Parent compound; 2-[6-(trifluoromethyl) pyridin-2-yloxymethyl]-benzoic
acid
(metabolite 8, soil); o-phthalic acid (metabolite 15, grain)
Ref: July
2003 - Review report for the active substance picoxystrobin.
Picoxystrobin SANCO/10196/2003-Final. 3 June 2003. Finalised
in the [European Commission] Standing Committee on the Food
Chain and Animal Health at its meeting on 4 July 2003 in
view of the inclusion of picoxystrobin in Annex I of Directive
91/414/EEC/.
http://www.fluorideaction.org/pesticides/picoxystrobin.eu.june.2003.pdf
|
Body
Weight Decrease
(click
on for all fluorinated pesticides)
--
Short term toxicity. Target / critical effect: No specific
target organ toxicity (rat, dog) Reduced
bodyweight and food consumption/ utilisation efficiency
at highest dose tested. Lowest relevant oral NOAEL / NOEL: 90-d
& 1-yr dietary, dog: 4.3 mg/kg bw/d. Lowest relevant dermal NOAEL
/ NOEL: 28-d, rat: >1000 mg/kg bw/d (limit dose)
-- Reproductive toxicity. Target / critical
effect - Reproduction: No effects on reproductive performance;
reduced body weights of offspring
at the end of the lactation period at parentally toxic doses (750
ppm). Lowest relevant reproductive NOAEL / NOEL: 2-gen, rat: >750
ppm (78.2 mg/kg bw/d), this being the highest dose tested; 200
ppm (parent rats) Target / critical effect
- Developmental toxicity: Ossification
delay and increased incidence of skeletal variants at maternally
toxic doses.
Lowest relevant developmental NOAEL / NOEL: rat: 30 mg/kg bw/day
rabbit: 25 mg/kg bw/day
-- Long term toxicity and carcinogenicity.
Target / critical effect: No significant target organ toxicity.
Reduced bodyweight and food consumption
at the highest dose tested (750 ppm). Lowest relevant NOAEL: 2-yr,
rat: 200 ppm (12.2 mg/kg bw/d). Carcinogenicity: No evidence of
carcinogenicity
-- Other
toxicological studies. o-phthalic
acid (Metabolite 15, grain): Survey of published literature
(November 2000) Acute oral LD50, rat: 7500-8400 mg/kg bw In-vitro
genotoxicity (Ames test & cytogenetic assay in CHO cells): negative.
Dominant lethal test: questionable positive test result involving
reduced male fertility and abnormal sperm morphology. Non-carcinogenic
in rats and mice according to NTP carcinogenicity programme. Reduced
foetal body weight and retarded ossification
in rats at maternal toxic doses
Ref:
July
2003 - Review report for the active substance picoxystrobin. Picoxystrobin
SANCO/10196/2003-Final. 3 June 2003. Finalised in the [European
Commission] Standing Committee on the Food Chain and Animal Health
at its meeting on 4 July 2003 in view of the inclusion of picoxystrobin
in Annex I of Directive 91/414/EEC/.
http://www.fluorideaction.org/pesticides/picoxystrobin.eu.june.2003.pdf
Bone
(click on for all fluorinated pesticides)
-- Reproductive
toxicity. Target / critical effect - Reproduction: No effects
on reproductive performance; reduced body
weights of offspring at the end of the lactation period
at parentally toxic doses (750 ppm). Lowest relevant reproductive
NOAEL / NOEL: 2-gen, rat: >750 ppm (78.2 mg/kg bw/d), this being
the highest dose tested; 200 ppm (parent rats) Target
/ critical effect - Developmental toxicity: Ossification
delay and increased incidence of skeletal variants
at maternally toxic doses. Lowest relevant developmental NOAEL
/ NOEL: rat: 30 mg/kg bw/day rabbit: 25 mg/kg bw/day
-- Other toxicological studies.
o-phthalic acid (Metabolite 15, grain): Survey of published
literature (November 2000) Acute oral LD50, rat: 7500-8400 mg/kg
bw In-vitro genotoxicity (Ames test & cytogenetic assay in CHO
cells): negative. Dominant lethal test:
questionable positive test result involving reduced male fertility
and abnormal sperm morphology. Non-carcinogenic in rats and mice
according to NTP carcinogenicity programme. Reduced foetal body
weight and retarded ossification
in rats at maternal toxic doses
Ref: July
2003 - Review report for the active substance picoxystrobin. Picoxystrobin
SANCO/10196/2003-Final. 3 June 2003. Finalised in the [European
Commission] Standing Committee on the Food Chain and Animal Health
at its meeting on 4 July 2003 in view of the inclusion of picoxystrobin
in Annex I of Directive 91/414/EEC/.
http://www.fluorideaction.org/pesticides/picoxystrobin.eu.june.2003.pdf
Endocrine:
Testicular (click
on for all fluorinated pesticides)
-- Other toxicological studies. o-phthalic acid (Metabolite 15,
grain): Survey of published literature (November 2000) Acute oral
LD50, rat: 7500-8400 mg/kg bw In-vitro genotoxicity (Ames test
& cytogenetic assay in CHO cells): negative. Dominant
lethal test: questionable positive test result involving reduced
male fertility and abnormal sperm morphology. Non-carcinogenic
in rats and mice according to NTP carcinogenicity programme. Reduced
foetal body weight and retarded ossification in rats at maternal
toxic doses
Ref: July
2003 - Review report for the active substance picoxystrobin. Picoxystrobin
SANCO/10196/2003-Final. 3 June 2003. Finalised in the [European
Commission] Standing Committee on the Food Chain and Animal Health
at its meeting on 4 July 2003 in view of the inclusion of picoxystrobin
in Annex I of Directive 91/414/EEC/.
http://www.fluorideaction.org/pesticides/picoxystrobin.eu.june.2003.pdf
|

