|
Return
to Penoxsulam Index Page
Activity:
Herbicide
(triazolopyrimidine)
Structure:
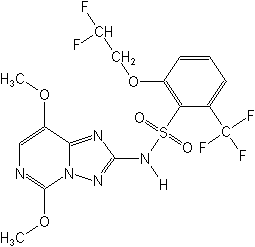
Adverse Effects:
Bladder
Body
Weight Decrease
Cancer: classified
as Suggestive Evidence of Carcinogenicity
(Mononuclear
cell leukemia)
Endocrine:
Testicular
Kidney
Leukemia
Liver
Skin
Environmental:
•
Penoxsulam
is expected to be very mobile in the environment with six degradation
products of toxicological concern to be even more mobile than the
parent compound.
• Data are not available to fully
characterize these degradates and their respective degradation pathways.
•
Potential
to leach to ground water
June 18, 2007 -
Penoxsulam. Human Health Risk Assessment for Proposed Uses
on Fish and Shellfish. Docket: EPA-HQ-OPP-2006-0076-0004.
USEPA.
Notes from FAN:
• This 84 page EPA document, which we have downloaded
for public access, has been deliberately "locked"
by EPA and can neither be searched nor sentences copied.
• EPA reported a finding in the rat developmental
toxicity study as Cutis laxis (pp 22-23), which we
can only assume is as rare a term as the condition itself.
If EPA persists in using terms that are not commonly used,
then they should provide a definition. An accessible definition
of Cutis Laxa was online: It is a rare disorder of
connective tissue that causes the skin to stretch easily
and hang in loose folds... Sometimes only the skin is affected,
but connective tissues throughout the body can be affected.
|
Bladder
(click on for all fluorinated pesticides)
-- Subchronic toxicity.
Dietary exposure to penoxsulam identified the
liver
and/or urinary tract (kidneys and
bladder) as target organs in rats, mice, and dogs following
a 4-week and 13-week administration. Effects on the liver were
reflected in increased liver weights and
hepatocellular hypertrophy, but these effects were not associated
with increases in mixed function oxidase (MFO) enzyme activity.
Effects noted in the kidneys included crystal deposition, most
likely from precipitation of penoxsulam from the urine, with resultant
irritation, inflammation, and hyperplasia of renal pelvic transitional
epithelium. Other than the crystal deposition in the kidneys,
all effects following subchronic exposure to rats appeared
to be reversible. Very high doses were associated with significant
decreases in body weight, weight gain, and feed consumption.
-- Chronic toxicity. Chronic exposure in the dog indicated that
the renal effects were not exacerbated with long-term exposure.
Following long-term exposure in rats, the
kidneys and urinary bladder were
the primary target organs. Histologic changes seen at the
end of 2 years of exposure consisted of inflammation and hyperplasia
of the renal pelvic transitional epithelium,
crystal deposition in the kidneys
and urinary bladder, and hyperplasia of the mucosa of the urinary
bladder...
Ref: August 6, 2003. Federal Register: August
6, 2003 (Volume 68, Number 151)] [Notices] [Page 46609-46613].
Penoxsulam; Notice of Filing a Pesticide Petition To Establish
a Tolerance for a Certain Pesticide Chemical in or on Food.
http://www.fluorideaction.org/pesticides/penoxsulam.fr.aug.6.2003.htm
In subchronic and chronic feeding studies
in rats and dogs, the most sensitive target organ was the urothelium
of the urinary system. Due to limited solubility in urine,
penoxsulam (and/or its metabolites) formed
crystals/calculi, which were regularly observed in the pelvis
of the kidney and the lumen of the urinary bladder. These crystals/calculi
apparently irritated the urothelium in these organs and following
repeated dosing lead to numerous secondary effects which resulted
in significant damage to the urinary system. In various
studies, these secondary effects were manifested as altered clinical
chemistry parameters (increased blood urea nitrogen), altered
urinalyses parameters (increased urine volume, decreased urine
specific gravity), increased absolute and relative kidney weights,
gross pathological findings in the kidneys (calculi and roughened
surface), and a variety of histopathological findings in the kidney
and urinary bladder, particularly hyperplasia, inflammation and
mineralization in the pelvic epithelium of the kidney and hyperplasia
in the mucosa of the urinary bladder.
Reference: September 2004. US EPA Fact Sheet
on Penoxsulam. Page 4.
http://www.fluorideaction.org/pesticides/penoxsulam.epa.fact.sheet.2004.pdf
Body
Weight Decrease
(click
on for all fluorinated pesticides)
(Pages
24-25) In a two-generation reproduction toxicity study (MRID 45830920),
XDE-638 (97.7% ai, lot #B-765-44, TSN102058) was administered
to 30 male and 30 female Crl:CD (SD) IGS BR rats/dose at dietary
concentrations that provided 0,30, 100, or 300 mg/kg/day. On litter
was produced in each generation. Fo and F1 parental animals were
administered test or control diet for 10 weeks prior to mating,
throughout mating, gestation, and lactation and until sacrifice...
Absolute body weights of the high-dose
F1 males were significantly (p≤0.05; 88-94% of controls)
less than those of the controls throughout the study...
Food consumption by the high-dose F1 males was significantly
less (p≤0.05; 92-93% of controls) than that of the
control group for the first two weeks of premating. Body weights
of the high-dose Fo and F1 dams were significantly
lower (p≤0.05; 87-94% of controls) than that of controls
from GD 21 through lactation day 14. The
most pronounced effect on body wieght gains during gestation was
for days 14-21 when the high-dose Fo and F1 dams had weight gains
79% and 82%, respectively, of the control group levels. Weight
changes by the high-dose dams during the first week of lactation
consisted of marked weight loss during days
1-4 and a lower weight gain than the controls for days
4-7.... Food consumption by the high-dose Fo dams was
significantly less (p≤0.05; 76-88% of controls) than
that of the controls on lactation days 1-11. Food consumption
by the high dose F1 dams was significantly
(p≤0.05; 70-72% of controls) less than that of the controls
on lactation days 1-7... High-dose male and female pups from both
generations had significantly lower (p≤0.05)
body weights on lactation days 4-21 compared with controls.
Ref: June 18, 2007 - Penoxsulam.
Human Health Risk Assessment for Proposed Uses on Fish and Shellfish.
Docket: EPA-HQ-OPP-2006-0076-0004. USEPA.
http://www.fluorideaction.org/pesticides/EPA-HQ-OPP-2006-0076-0004.pdf
-- Subchronic toxicity.
Dietary exposure to penoxsulam identified the
liver and/or urinary tract (kidneys and bladder) as target organs
in rats, mice, and dogs following a 4-week and 13-week administration.
Effects on the liver were reflected in increased liver weights
and hepatocellular hypertrophy, but these effects were not associated
with increases in mixed function oxidase (MFO) enzyme activity.
Effects noted in the kidneys included crystal deposition, most
likely from precipitation of penoxsulam from the urine, with resultant
irritation, inflammation, and hyperplasia of renal pelvic transitional
epithelium. Other than the crystal deposition in the kidneys,
all effects following subchronic exposure to rats appeared
to be reversible. Very high doses were associated
with significant decreases in body weight, weight gain, and feed
consumption.
-- Reproductive and developmental toxicity. Penoxsulam
did not have any effect on reproductive parameters at dose levels
that induced treatment-related effects in parental rats. At the
highest dosage tested (HDT) (300 mg/kg/day), body
weights and weight gains in both males and females were depressed,
liver and/or kidney weights were increased, and histologic
changes were noted in the liver
(males) and kidneys (females).
At 100 mg/kg/day, increased liver weights were recorded in males,
with no histologic correlate, and histologic changes noted in
the kidneys of females. Transient
decreases in pup body weights were seen at the HDT, but
dietary concentrations were targeted for adults and consumption
of treated diets by the pups resulted in dose levels to the pups
approximately 3-fold higher than in adults. A teratogenic potential
was not demonstrated for penoxsulam in either rats or rabbits.
Ref: August 6, 2003. Federal Register: August
6, 2003 (Volume 68, Number 151)] [Notices] [Page 46609-46613].
Penoxsulam; Notice of Filing a Pesticide Petition To Establish
a Tolerance for a Certain Pesticide Chemical in or on Food.
http://www.fluorideaction.org/pesticides/penoxsulam.fr.aug.6.2003.htm
Cancer
(click
on for all fluorinated pesticides)
Suggestive
Evidence of Carcinogenicity, but
Not Sufficient to Assess Human Carcinogenic Potential. Mononuclear
cell leukemia
in Male Fischer 344 rats. Although dosing in male mice was not
considered to be adequate, an additional mouse carcinogenicity
study was not required.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
--
Carcinogenicity: Evidence of carcinogenicity
in male rats based on possibly treatment related increase incidence
of Large Granular Lymphocyte (LGL) Leukemia at 5, 50, & 250
mg/kg/day. Also
increase severity at 250 mg/kg/day. Female rats - negative for
carcinogenicity, but dosing was only marginally adequate.
-- Carcinogenicity-mice: In males, negative for carcinogenicity
at doses tested. Dosing inadequate.
-- The Agency (US EPA) has
classified penoxsulam as Suggestive Evidence of Carcinogenicity,
But not sufficient to assess human carcinogenic
potential and, therefore, quantification of human cancer risk
is not required. The weight-of-the-evidence for this classification
is as follows: a. Evidence of carcinogenicity (mononuclear cell
leukemia (MNCL)) was seen in one sex (males) of one species (rat).b.
There was an increased incidence of MNCL at all dose levels with
all incidences exceeding the laboratory historical control, however,
the dose-response was flat over a wide range of doses. c. Although
MNCL is recognized as a common neoplasm in Fischer rats, the mechanism
of producing MNCL is not completely understood. Therefore, the
significance of MNCL and its biological relevance for human cancer
risk remains uncertain and cannot be discounted ...
Ref: September 24, 2004. Penoxsulam Pesticide
tolerance. Final Rule. Federal Register.
http://www.fluorideaction.org/pesticides/penoxsulam.fr.sept.24.04.htm
Endocrine:
Testicular (click
on for all fluorinated pesticides)
--
Significant dose-related effects in the two-generation reproduction
study were limited to the delay in preputial separation.
-- 2-Generation Reproduction and fertility
effects in rats - Reproductive/Offspring LOAEL = 100 mg/kg/day
based on delayed preputial separation
Ref:
September 24, 2004. Penoxsulam Pesticide tolerance. Final Rule.
Federal Register.
http://www.fluorideaction.org/pesticides/penoxsulam.fr.sept.24.04.htm
-- Preputial
separation, an indicator of sexual maturation, was significantly
(p#0.05) delayed in mid and high dose F1 males. The mean
age at which preputial separation was attained for the control,
low, mid, and high dose groups was 43.6, 44.0, 45.5, and 46.0
days, respectively. In addition, at the mid dose, 1 animal did
not separate and at the high dose, 3 animals did not separate
whereas all animals at the control and low doses did separate.
The delay in preputial separation at the
mid and high doses was considered to be a treatment-related effect.
-- Endocrine Disruption. For penoxsulam, effects which indicate
potential endocrine disruption include kidney lesions (crystals)
in female rats and delay in preputial separation in male rats.
When the appropriate screening and/or testing
protocols being considered under the Agency‘s EDSP have
been developed, penoxsulam may be subjected to additional screening
and/or testing to better characterize effects related to endocrine
disruption.
Reference: September 2004. US EPA Fact Sheet
on Penoxsulam. Page 4.
http://www.fluorideaction.org/pesticides/penoxsulam.epa.fact.sheet.2004.pdf
Kidney
(click
on for all fluorinated pesticides)
-- Subchronic toxicity.
Dietary exposure to penoxsulam identified the
liver and/or urinary tract (kidneys
and bladder)
as target organs in rats, mice, and
dogs following a 4-week and 13-week administration... Effects
noted in the kidneys included crystal
deposition, most likely from precipitation of penoxsulam from
the urine, with resultant irritation, inflammation, and hyperplasia
of renal pelvic transitional epithelium. Other
than the crystal deposition in the kidneys, all effects
following subchronic exposure to rats appeared to be reversible...
-- Chronic toxicity. Chronic exposure in the dog indicated that
the renal effects were not exacerbated with long-term exposure.
Following long-term exposure in rats, the kidneys
and urinary bladder were the primary
target organs. Histologic changes seen at the end of 2
years of exposure consisted of inflammation and hyperplasia of
the renal pelvic transitional epithelium,
crystal deposition in the kidneys and urinary bladder, and hyperplasia
of the mucosa of the urinary bladder...
-- Reproductive and developmental toxicity. Penoxsulam did not
have any effect on reproductive parameters at dose levels that
induced treatment-related effects in parental rats. At the highest
dosage tested (HDT) (300 mg/kg/day), body
weights and weight gains in both males and females were depressed,
liver and/or kidney weights were
increased, and histologic changes were noted in the
liver (males) and kidneys (females).
At 100 mg/kg/day, increased liver weights
were recorded in males, with no histologic correlate, and
histologic changes noted in the kidneys of females...
Ref: August 6, 2003. Federal Register: August
6, 2003 (Volume 68, Number 151)] [Notices] [Page 46609-46613].
Penoxsulam; Notice of Filing a Pesticide Petition To Establish
a Tolerance for a Certain Pesticide Chemical in or on Food.
http://www.fluorideaction.org/pesticides/penoxsulam.fr.aug.6.2003.htm
-- Endocrine Disruption. For penoxsulam, effects which indicate
potential endocrine disruption include kidney
lesions (crystals) in female rats and delay in preputial
separation in male rats. When the appropriate
screening and/or testing protocols being considered under the
Agency‘s EDSP have been developed, penoxsulam may be subjected
to additional screening and/or testing to better characterize
effects related to endocrine disruption.
Reference: September 2004. US EPA Fact Sheet
on Penoxsulam. Page 4.
http://www.fluorideaction.org/pesticides/penoxsulam.epa.fact.sheet.2004.pdf
Leukemia
(click on for all fluorinated pesticides)
Suggestive
Evidence of Carcinogenicity, but
Not Sufficient to Assess Human Carcinogenic Potential. Mononuclear
cell leukemia
in Male Fischer 344 rats. Although dosing in male mice was not
considered to be adequate, an additional mouse carcinogenicity
study was not required.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
--
Carcinogenicity: Evidence of carcinogenicity
in male rats based on possibly treatment related increase incidence
of Large Granular Lymphocyte (LGL) Leukemia at 5, 50, & 250
mg/kg/day. Also
increase severity at 250 mg/kg/day. Female rats - negative for
carcinogenicity, but dosing was only marginally adequate.
-- Carcinogenicity-mice: In males, negative for carcinogenicity
at doses tested. Dosing inadequate.
-- The Agency (US EPA) has
classified penoxsulam as Suggestive Evidence of Carcinogenicity,
But not sufficient to assess human carcinogenic
potential and, therefore, quantification of human cancer risk
is not required. The weight-of-the-evidence for this classification
is as follows: a. Evidence of carcinogenicity (mononuclear cell
leukemia (MNCL)) was seen in one sex (males) of one species (rat).b.
There was an increased incidence of MNCL at all dose levels with
all incidences exceeding the laboratory historical control, however,
the dose-response was flat over a wide range of doses. c. Although
MNCL is recognized as a common neoplasm in Fischer rats, the mechanism
of producing MNCL is not completely understood. Therefore, the
significance of MNCL and its biological relevance for human cancer
risk remains uncertain and cannot be discounted ...
Ref: September 24, 2004. Penoxsulam Pesticide
tolerance. Final Rule. Federal Register.
http://www.fluorideaction.org/pesticides/penoxsulam.fr.sept.24.04.htm
-- Chronic toxicity.
Chronic exposure in the dog indicated that the renal effects were
not exacerbated with long-term exposure. Following long-term exposure
in rats, the kidneys and urinary bladder
were the primary target organs. Histologic changes seen at the
end of 2 years of exposure consisted of inflammation and hyperplasia
of the renal pelvic transitional epithelium, crystal deposition
in the kidneys and urinary bladder, and hyperplasia of the mucosa
of the urinary bladder. In
the mouse, the liver was the primary target organ, and histologic
changes consisted of hepatocellular hypertrophy. There were no
treatment-related increases in tumors in either rats or mice.
The
incidence of mononuclear cell leukemia (Fischer rat leukemia)
was increased in all groups of treated male
rats compared to the concurrent controls. However, the
incidences in the treated groups were identical across a 50-fold
increase in dosage, and well within the range of control values
reported in the literature.
Ref: August 6, 2003. Federal Register: August
6, 2003 (Volume 68, Number
151)] [Notices] [Page 46609-46613]. Penoxsulam; Notice of Filing
a Pesticide Petition To Establish a Tolerance for a Certain Pesticide
Chemical in or on Food.
http://www.fluorideaction.org/pesticides/penoxsulam.fr.aug.6.2003.htm
In a carcinogenicity study in rats, male and female rats were
given penoxsulam in the diet for two years at dose levels of 0,
5, 50 or 250 mg/kg/day. In this study, there
was a statistically significant increased incidence of malignant
large granular lymphocyte (LGL) leukemia in each of the male treatment
groups. The incidence was 24%, 60%, 58% and 60% in the
control, low, mid and high dose level groups respectively. There
was no dose response with all treated male groups having an approximately
2.5 fold increase over control animals. The
incidence in the male treatment groups exceeded the conducting
laboratory‘s historical control mean (28.5%) and range (16-40%),
but fell within the National Toxicology Program (NTP) historical
control data base of mean (50.5%) and range (32-74 %). There
was also an increased severity (Stage 3) of LGL leukemia in all
the treated male groups compared to the control group.
There was no increase in incidence or severity of LGL leukemia
for the treated female rats in this study. The dose levels in
this study were considered to be adequate in male rats and marginally
adequate in female rats to assess the carcinogenicity of penoxsulam.
Reference: September 2004. US EPA Fact Sheet
on Penoxsulam. Page 4.
http://www.fluorideaction.org/pesticides/penoxsulam.epa.fact.sheet.2004.pdf
Liver
(click on for all fluorinated pesticides)
-- Subchronic toxicity.
Dietary exposure to penoxsulam identified the
liver and/or urinary tract (kidneys
and bladder) as target organs in rats, mice, and dogs following
a 4-week and 13-week administration. Effects on the liver were
reflected in increased liver weights and hepatocellular
hypertrophy, but these effects were not associated with
increases in mixed function oxidase (MFO) enzyme activity... Other
than the crystal deposition in the kidneys, all effects
following subchronic exposure to rats appeared to be reversible.
Very high doses were associated with significant decreases in
body weight, weight gain, and feed consumption.
-- Reproductive and developmental toxicity. Penoxsulam did not
have any effect on reproductive parameters at dose levels that
induced treatment-related effects in parental rats. At the highest
dosage tested (HDT) (300 mg/kg/day), body
weights and weight gains in both males and females were depressed,
liver and/or kidney weights were
increased, and histologic changes were noted in the
liver (males) and kidneys (females). At 100 mg/kg/day,
increased liver weights were recorded in
males, with no histologic correlate, and
histologic changes noted in the kidneys of females...
Ref: August 6, 2003. Federal Register: August
6, 2003 (Volume 68, Number 151)] [Notices] [Page 46609-46613].
Penoxsulam; Notice of Filing a Pesticide Petition To Establish
a Tolerance for a Certain Pesticide Chemical in or on Food.
http://www.fluorideaction.org/pesticides/penoxsulam.fr.aug.6.2003.htm
Skin
(click
on for all fluorinated pesticides)
(Page 22-23): In a developmental toxicity study (MRID 45830917)
XDE-638 (Penoxsulam; 97.5% ai, lot #ND05167938, TSN101773) was
administered to 25 time-mated female CD rats/dose by gavage in
0.5% aqueous METHOCEL at dose levels of 0, 100, 500, or 1000 mg/kg
bw/day on gestation days (GD) 6 through 20, inclusive. On GD 21,
surviving females were sacrificed and necropsied. All fetuses
were weighed sexed, and examined for external alterations. Approximately
one-half of the fetuses from each litter were subjected to visceral
examination, and the remaining one-half were subjected to skeletal
examinaiton... Malformations were observed in 0, 2, 2 and 3 fetuses
and in 0/24, 2/24, 1/25, and 2/22 litters from the control, low-,
mid-, and high-dose groups, respectively.... An
apparently rare external malformation (cutis laxis*) was observed
in 2 fetuses in single litters at both the 500 and 1000
mg/kg/day dose levels. However, based on a weight-of-the-evidence
consideration of all the available information/data, it is concluded
that the cutis laxis observed inthis study most likely has a genetic
etiology. There is insufficient information to conclude that it
is a treatment-related effect due to the test material. Therefore,
the development toxicity LOAEL for penoxsulam in CD rats is not
identied (>1000 mg/kg day), and the developmental toxicity
NOAEL is 1000 mg/kg day.
Ref: June 18, 2007 - Penoxsulam.
Human Health Risk Assessment for Proposed Uses on Fish and Shellfish.
Docket: EPA-HQ-OPP-2006-0076-0004. USEPA.
http://www.fluorideaction.org/pesticides/EPA-HQ-OPP-2006-0076-0004.pdf
NOTE FROM FAN: We could not find the term
cutix laxis, only the term Cutis Laxa.
* Definition of Cutis laxa: Pathophysiology: Cutix laxa
is characterized by degenerative changes in the elastic fibers
resulting in loose, pendulous skin. The skin is sagging, redundant,
and stretchable, with reduced elastic recoil.
Ref: eMedicine site: Cutis Laxa (Elastolysis).
http://www.emedicine.com/derm/topic93.htm
from Merck.com: : Cutis laxa is a
rare disorder of connective tissue that causes the skin to stretch
easily and hang in loose folds... Sometimes only the skin is affected,
but connective tissues throughout the body can be affected. Cutis
laxa is usually hereditary. In some kinds of cutis laxa, the abnormal
genes cause problems unrelated to connective tissues - for example,
mental retardation....
http://www.merck.com/mmhe/sec23/ch279/ch279e.html
| ENVIRONMENTAL
(click
on for all fluorinated pesticides)
(page 8): Penoxsulam
is expected to be very mobile in the environment with the
degradation products of toxicological concern to be even
more mobile than the parent compound.
(Page 39): Penosxulam is stable to hydrolysis,
and is expected to be somewhat persistent in non-aquatic
environments. The major route of dissipation for penoxsulam
in clear and shallow surface water under favorable light
conditions is through direct acqueous photolysis (t 1/2=1.5-14
days). Penoxsulam is slightly more persistent in aerobic
aquatic (t 1/2 = 12-38 days) and anaerobic
environments (t 1/2 = 34-118 days). Penoxsulam is
also very mobile (Kd=0.13-1.96), and does
have the potential to leach to ground water. The
low vapor pressure and Henry's Law constant, limits the
potential of penoxsulam to volatilization from soil and
water.
Eleven major degradation products have been
identified for penoxsulam. Data are not available to fully
characterize these degradates and their respective degradation
pathways. Six of these degradation
products have been identified by HED as being of toxicological
concern. These toxic residues are:
BSTCA
2-animo TCA
5-OH-penoxsulam
SFA
sulfonamide
5,8-di-OH-penoxsulam
Ref: June 18, 2007
- Penoxsulam. Human Health Risk Assessment for Proposed
Uses on Fish and Shellfish. Docket: EPA-HQ-OPP-2006-0076-0004.
USEPA.
http://www.fluorideaction.org/pesticides/EPA-HQ-OPP-2006-0076-0004.pdf
|
|

