|
Return
to Noviflumuron Index Page
Activity:
Insecticide
(Benzoylurea)
Structure:
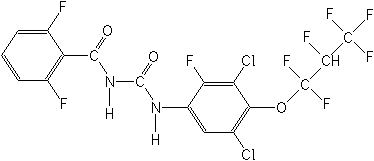
Adverse Effects:
Blood
Body Weight Decrease
Bone
Brain
Cancer: LIVER, LUNG
Endocrine: Adrenal
Endocrine: Testicular
Endocine: Uterine
Eye
Kidney
Liver
Lung
Reproductive
Spleen
Stomach
Teratogen
Environmental: highly
toxic to freshwater invertebrates
•
Note: As of October 12, 2003, very little toxicological
data is available.
•
Noviflumuron is a structural analog of the pesticide active
ingredient hexaflumuron
[and Novaluron
(EC)]. Pesticide products containing hexaflumuron are currently
registered for use in California for termite control. However,
recent research indicates noviflumuron is more efficacious
than hexaflumuron and Dow AgroSciences LLC plans to eventually
replace it with noviflumuron. As an insect growth regulator
(IGR), noviflumuron prevents the successful molting and
development of subterranean termites and eventually eliminates
the colony. Products formulated with noviflumuron are intended
for use only when termite activity is detected...
Ref: 2003.
Noviflumuron.
California Department of Pesticide Regulation. Public Report
2003-5.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2003.pdf
|
Blood
and Cholesterol
(click on for all fluorinated
pesticides)
-- “XDE-007: One-Year Dietary Toxicity
Study in Beagle Dogs,” (Stebbins, K.E., Day, S.J.,
Thomas, J.; Toxicology & Environmental Research and Consulting,
The Dow Chemical Company, Midland, MI; Laboratory Project Study
ID #: 142640; 3/5/04). XDE-007 (97.9% pure) was fed in diet to
Beagle dogs (4/sex/dose) for 1 year at 0, 0.003, 0.03 or 0.225%
(equivalent to 0, 0.74, 9.3 and 69 mg/kg/day - Males and 0, 0.94,
8.7 and 70 mg/kg/day - females)... Both
sexes at > 0.225% had statistically significantly increased
mean platelet count. There was a significant increase in ALP at
0.225% in females at 3 and 12 months.
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
-- 007; 186499; "XDE-007: 28-Day Dietary
Toxicity Study in CD-1 Mice" (Yano, B.L. and Day,
S.J., Toxicology & Environmental Research and Consulting, The
Dow Chemical Company, Midland,
MI, Laboratory Project Study ID 001248, 6/12/01). XDE-007 (Lot,
Reference No. F0031-148, TSN102332, purity = 98.4%) was admixed
to the feed and fed to 5 CD-1 mice per sex per dose at dose levels
of 0, 10, 100, 500, or 1000 mg/kg/day (0, 10.8, 110, 538, 1060
mg/kg/day, respectively for males and 0, 11.2, 113, 504, 1140
mg/kg/day, respectively for females) for 28 days. No mortalities
occurred. No treatment-related clinical signs were observed. Treatment-related
increases in mean platelet level
and mean cholesterol level were observed in both sexes
at 100, 500, and 1000 mg/kg/day. A treatment-related increase
in mean relative liver weight was observed
at in males at 100, 500, and 1000 mg/kg/day and in females at
500 and 1000 mg/kg/day. Microscopic examination revealed treatment-related
hepatocellular hypertrophy with altered tinctorial properties
(centrilobular/midzonal to panlobular) in males at 500 and 1000
mg/kg/day and very slight vacuolation (consistent with fatty change)
of the periportal hepatocytes in males at 500 and 1000
mg/kg/day and in females at 1000 mg/kg/day. No adverse effects.
NOEL (M) = 10.8 mg/kg/day and NOEL (F) = 11.2 mg/kg/day (based
on increases in mean
platelet and mean cholesterol levels). Supplemental (because
only 5 animals per sex per dose were used and because the animals
were treated for only 28 days). (Corlett, 9/30/02)
Ref: September 26, 2002. Summary of Toxicology
Data for Noviflumuron ((XDE-007) or N-(((3,5-dichloro-2-fluoro-4-(1,1,2,3,3,3-hexafluoropropoxy)phenyl)amino)carbonyl)-2,6-
diflurobenzamide. California EPA, Department of Pesticide Regulation,
Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Noviflumuron.CA.EPA.2002.pdf
Body
Weight Decrease (click
on for all fluorinated pesticides)
-- “XDE-007: One-Generation Dietary
Reproduction Toxicity Study With CrossFostering in CD Rats,”
(Marty, M.S., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology
& Environmental Research and Consulting, The Dow Chemical
Company, Midland, MI; Laboratory Project Study ID: 011221; 3/23/04).
XDE -007 (97.9% pure) was fed in diet to Crl:CD (SD) IGS BR rats
(30/sex/dose) at 0, 0.5, 5 and 100 mg/kg/day continuously from
pre-mating (10 weeks) of parental generation, through breeding
(2 weeks), gestation (3 weeks) and lactation through weaning of
F1 offspring. Although designed as a 2 generation reproduction
study, it was terminated after 1 generation due to excessive F1
pup mortality at 100 mg/kg/day. Subsequently a Cross-fostering
Study was performed to determine whether the decreased survival
in pups from XDE-007 treated P1 rats resulted from in utero or
lactational exposure. Parental Systemic NOEL = 0.5 mg/kg/day (Tonoclonic
convulsions were observed in 2/30 males and 1/24 females at 100
mg/kg/day. Males had statistically significantly decreased food
consumption from day 8 until termination at 100 mg/kg. Females
had statistically significantly decreased food consumption during
lactation. Male P1 premating body weights
were statistically significantly decreased at 100 mg/kg/day. Mean
gestational body weight gain in females was statistically significantly
decreased GD 0 - 7 (but not GD 0 - 21) and lactation days
7 - 13 at 100 mg/kg/day.) Reproduction and Fertility NOEL = 0.5
mg/kg/day (P1 female gestation survival was decreased and gestation
length was increased at 100 mg/kg. Pup NOEL = 0.5 mg/kg (F1 survival
was drastically decreased throughout lactation at 100 mg/kg. Clinical
effects were increased in pups at 100 mg/kg, primarily tonoclonic
convulsions, no milk in stomach, entire litter loss and death.
Pup weights were statistically significantly
decreased at 100 mg/kg. Cross-fostering data indicate the
proximate toxicant (either XDE-007 and/or its metabolites) led
to decreased pup survival through the maternal milk and not through
exposure in utero. Possible adverse effect indicated: excessive
neonatal/pup mortality and increased tonoclonic convulsions in
pups (17/24 litters) at 100 mg/kg. These data are supplemental.
M. Silva, 7/20/04
-- REPRODUCTION, RAT. “XDE-007: Two-Generation
Dietary Reproduction Toxicity Study in CD Rats,”
(Carney, E.W., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology
& Environmental Research and Consulting, The Dow Chemical
Company, Midland, MI; 3/22/04). XDE -007 (97.9% pure) was fed
in diet to Crl:CD (SD) IGS BR rats (30/sex/dose/generation) at
0, 0.5, 5 or 25 mg/kg/day continuously from pre-mating of parental
generation 1 (P1) through weaning of offspring through 2 generations
(2 matings for P2 generation) to F2b weaning... Mean
F2a litter size was statistically significantly decreased
on lactation days 14 and 21 at 25 mg/kg. Mean
F1 pup weights were statistically significantly decreased at
25 mg/kg on lactation days 1, 14 and 21. Mean
F2a male pup weights were statistically significantly decreased
on lactation days 1 and 21 and F2a female pup weights were
decreased on lactation day 21 at 25 mg/kg. F2a
male weanlings had statistically significantly decreased body
weights, relative brain and absolute spleen
weights at 25 mg/kg. Neonates
at 25 mg/kg in both F1 and F2 generations showed tonoclonic convulsions,
as well as in 1 litter of F2a at 5.0 mg/kg. P2 females (1, 1,
5 and 7 at 0, 0.5, 5.0 and 25 mg/kg, respectively) failed
to litter, so the female is considered to be an affected sex.)
Possible adverse effects on reproduction, fertility and pup survival,
along with numerous other toxicologically relevant effects. M.
Silva, 6/18/04
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Bone
(click
on for all fluorinated pesticides)
-- “XDE-007: 90-Day Dietary Toxicity
Study with 28-Day Recovery in Beagle Dogs,” (Stebbins,
K.E., Thomas, J., Baker, P.C.; Toxicology & Environmental
Research and Consulting, The Dow Chemical Company, Midland, MI;
Laboratory Project Study ID #: 011194; 5/9/03). XDE-007 (98.4%
pure) was fed in diet to Beagle dogs (4/sex/dose) for 90 days
at 0, 0.003, 0.3 or 3.0% (equivalent to 0, 0.913, 115 and 1040
mg/kg/day - Males and 0, 1.06, 113 and 1150 mg/kg/day - females).
Recovery groups (4/sex/dose) were fed XDE-007 in diet at 0 or
3% for 90 days, then were maintained on control diet for 28 days.
... There was an increased incidence in
bone marrow hyperplasia (erythroid cell) at > 0.3% XDE-007
in the main dose group that persisted after recovery.
-- “XDE-007: One-Year Dietary Toxicity
Study in Beagle Dogs,” (Stebbins, K.E., Day, S.J.,
Thomas, J.; Toxicology & Environmental Research and Consulting,
The Dow Chemical Company, Midland, MI; Laboratory Project Study
ID #: 142640; 3/5/04). XDE-007 (97.9% pure) was fed in diet to
Beagle dogs (4/sex/dose) for 1 year at 0, 0.003, 0.03 or 0.225%
(equivalent to 0, 0.74, 9.3 and 69 mg/kg/day - Males and 0, 0.94,
8.7 and 70 mg/kg/day - females)... There
was an increased incidence in bone marrow erythroid hyperplasia
(erythroid cell) at > 0.3% XDE-007 (males, 0, 0, 4, 4 --each
dose level) and at 0.225% (females: 0, 0, 0, 1 --each dose level).
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Brain
(click on for all fluorinated
pesticides)
-- REPRODUCTION, RAT. “XDE-007: Two-Generation
Dietary Reproduction Toxicity Study in CD Rats,”
(Carney, E.W., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology
& Environmental Research and Consulting, The Dow Chemical
Company, Midland, MI; 3/22/04). XDE -007 (97.9% pure) was fed
in diet to Crl:CD (SD) IGS BR rats (30/sex/dose/generation) at
0, 0.5, 5 or 25 mg/kg/day continuously from pre-mating of parental
generation 1 (P1) through weaning of offspring through 2 generations
(2 matings for P2 generation) to F2b weaning. . ... . F2a
male weanlings had statistically significantly decreased body
weights, relative brain
and absolute spleen weights at 25
mg/kg. Neonates at
25 mg/kg in both F1 and F2 generations showed tonoclonic convulsions,
as well as in 1 litter of F2a at 5.0 mg/kg. P2 females (1, 1,
5 and 7 at 0, 0.5, 5.0 and 25 mg/kg, respectively) failed
to litter, so the female is considered to be an affected sex.)
Possible adverse effects on reproduction, fertility and pup survival,
along with numerous other toxicologically relevant effects. M.
Silva, 6/18/04
-- “XDE-007: Two-year Dietary Chronic
Toxicity/Oncogenicity and Chronic Neurotoxicity Study in Fischer
344 Rats,” (Yano, B.L., Dryzga, M.D., Thomas, J.;
Toxicology & Environmental Research and Consulting, The Dow
Chemical Company, Midland, MI; Laboratory Project Study ID: 011168;
4.22.05). Noviflumuron ((N-[3,5-dichloro-2-fluoro-4(1,1,2,3,3,3-hexafluoropropoxy)phenyl]-N’-(2,6-difluorobenzoyl)urea;
XDE-007, 97.9% pure) was fed in diet to Fischer 344 rats (75/sex/dose)
for 90 days, 1 or 2 years at 0, 0.1, 1.0, 75 or 300 mg/kg/day.
Subchronic toxicity was assessed after 90 days of treatment on
10/sex/dose at 0 and 1.0 mg/kg/day doses only. At 1 year,10/sex/dose
(chronic toxicity group), and 5/sex/dose (chronic neurotoxicity
group) were necropsied. The remaining 50/sex/dose were necropsied
after 2 years (oncogenicity group). Chronic NOEL = 1.0 mg/kg/day
(At > 75 mg/kg/day there were decreased body weights and body
weight gains along with increases or decreases
in absolute and/or relative organ weights (liver, kidney,
brain, heart, adrenal, testes, spleen,
epididymides were affected) at 12 and/or 24 months.
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
-- 52905-009 186501,
"XR-007: Whole Embryo Culture Teratogenicity
Screen", (E. W. Carney, Health and Environmental Research
Laboratories, The Dow Chemical Company,
Midland, MI, Report # 971083, 31 July 1997). Seven non-pregnant
female rats (serum donors) received 1000 mg/kg/day of the test
article (XR-007, 98%) in 0.5% Methocel by gavage for 3 consecutive
days. Four hours after the last dose, rats were exsanguinated
and their blood centrifuged to obtain serum. Six control rats
were similarly treated with vehicle and bled...
Statistically significant increases in crown-rump length and somite
number for treated embryos were reportedly due to a lower
than usual growth rate in control embryos. Morphological
abnormality was limited to an abnormal curvature of the anterior
neural tube which distorted the head in one treated embryo
(8.3%). No historical control data. This is supplemental information.
(Green and Gee, 10/3/02).
Ref:
September 26, 2002. Summary of Toxicology Data for Noviflumuron
((XDE-007) or N-(((3,5-dichloro-2-fluoro-4-(1,1,2,3,3,3-hexafluoropropoxy)phenyl)amino)carbonyl)-2,6-
diflurobenzamide. California EPA, Department of Pesticide Regulation,
Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Noviflumuron.CA.EPA.2002.pdf
[Note
from FAN- some definitions:
"Predominately anterior neural tube
defects, occuring early in gestation. The anterior neural
tube fails to close properly, or does not divide into two hemispheres.
The generic name for this category of malformations is arrhinencephaly.
Ref: Developmental and Perinatal Disorders of the CNS. Weill
Medical College of Cornell University. http://edcenter.med.cornell.edu/CUMC_PathNotes/Neuropathology/Neuropath_II/dev2.html
-- "... trisomy 13 is closely linked to midline malformations,
including arrhinencephaly,
agenesis of the corpus callosum, and holoprosencephaly, and
is the single most common chromosomal defect underlying holoprosencephaly."
- http://128.100.71.82/neurosurgery/developmental.html
-- "Trisomy 13 Syndrome is
a genetic disorder with onset before birth. It occurs approximately
1/5000 live births. Infants affected with Trisomy 13 tend
to be small at birth. Spells of interrupted breathing (apnea)
in early infancy are frequent, and mental retardation is usually
severe. Many affected children appear to be deaf. A moderately
small head (microcephaly) with sloping forehead, wide joints
and openings between parietal bones of the head are present.
Gross anatomic defects of the brain, especially failure of the
forebrain to divide properly (holoprosencephaly) are common.
A hernial protrusion of the cord and its meninges through a
defect in the vertebral canal (myelomeningocele) is found in
almost 50% of cases. The entire eye is usually small (microphthalmia),
and a defect of the iris tissue (coloboma), and faulty development
of the retina (retinal dysplasia) occur frequently. The supraorbital
ridges are shallow and palapebral fissures are usually slanted.
Cleft lip, cleft palate, or both are present in most cases.
The ears are abnormally shaped and unusually low-set."
-
http://www.trisomy.org/html/trisomy_13_facts.htm
Cancer
(click
on for all fluorinated pesticides)
“XDE-007: 18-Month Oncogenicity Study
in CD-1 Mice,” (Johnson, K.A.; Toxicology & Environmental
Research and Consulting, The Dow Chemical Company, Midland, MI;
4/25/05). XDE-007 technical (N-[3,5-dichloro-2-fluoro-4-(1,1,2,3,3,3hexafluoropropoxy)phenyl]-N’-(2,6-difluorobenzoyl)urea),
97.9% pure) was fed in diet to CD-1 mice (50/sex/dose) at 0, 0.5,
3 (males only), 30 and 100 (females only) mg/kg/day for up to
18 months. Systemic NOEL = 3 mg/kg/day (males) and 30 mg/kg/day
(females) There was an increase in mortality for females at 100
mg/kg/day (M: 56% vs 32%). There was an increased incidence in
tonoclonic convulsions (primarily in females) at the high dose.
There was an increase in relative and absolute liver weights in
females at > 30 mg/kg/day. Males had an absolute liver weight
increase at 30 mg/kg/day and a relative increase at > 3 mg/kg/day.
Both sexes showed an increase in all severities of liver hypertrophy
(hepatocytic, centrilobular/midzonal, diffuse) at 30 mg/kg/day
(male) and at 100 mg/kg/day (female)...
Possible adverse effect indicated: There
was an increased incidence in hepatocytic adenomas and in hepatocytic
adenomas plus carcinomas (slight in males, statistically
significant in females) at 30 mg/kg/day (5, 7, 4, 12
of 50) and 100 mg/kg/day (F: 0, 1, 0, 4**
of 50 by Peto’s mortality adjusted statistics ).
Females showed an increased incidence in lung carcinomas
(non-metastatic) at 100 mg/kg/day (0, 0, 1, 2** by Peto’s
statistics).
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Endocrine:
Adrenal (click
on for all fluorinated pesticides)
-- “XDE-007: One-Year Dietary Toxicity
Study in Beagle Dogs,” (Stebbins, K.E., Day, S.J.,
Thomas, J.; Toxicology & Environmental Research and Consulting,
The Dow Chemical Company, Midland, MI; Laboratory Project Study
ID #: 142640; 3/5/04). XDE-007 (97.9% pure) was fed in diet to
Beagle dogs (4/sex/dose) for 1 year at 0, 0.003, 0.03 or 0.225%
(equivalent to 0, 0.74, 9.3 and 69 mg/kg/day - Males and 0, 0.94,
8.7 and 70 mg/kg/day - females)... There
was a statistically significant increase in absolute adrenal weights
(analyzed across both sexes) at 0.225% without histological findings.
-- “XDE-007: One-Generation Dietary
Reproduction Toxicity Study With CrossFostering in CD Rats,”
(Marty, M.S., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology
& Environmental Research and Consulting, The Dow Chemical
Company, Midland, MI; Laboratory Project Study ID: 011221; 3/23/04).
XDE -007 (97.9% pure) was fed in diet to Crl:CD (SD) IGS BR rats
(30/sex/dose) at 0, 0.5, 5 and 100 mg/kg/day continuously from
pre-mating (10 weeks) of parental generation, through breeding
(2 weeks), gestation (3 weeks) and lactation through weaning of
F1 offspring. Although designed as a 2 generation
reproduction study, it was terminated after 1 generation due to
excessive F1 pup mortality at 100 mg/kg/day. Subsequently
a Cross-fostering Study was performed to determine whether the
decreased survival in pups from XDE-007 treated P1 rats resulted
from in utero or lactational exposure. Parental Systemic NOEL
= 0.5 mg/kg/day (Tonoclonic convulsions were observed in 2/30
males and 1/24 females at 100 mg/kg/day. Males had statistically
significantly decreased food consumption from day 8 until termination
at 100 mg/kg. Females had statistically significantly decreased
food consumption during lactation. Male
P1 premating body weights were statistically significantly decreased
at 100 mg/kg/day. Mean gestational body
weight gain in females was statistically significantly decreased
GD 0 - 7 (but not GD 0 - 21) and lactation days 7 - 13 at 100
mg/kg/day.) Reproduction and Fertility NOEL = 0.5 mg/kg/day (P1
female gestation survival was decreased and gestation length was
increased at 100 mg/kg. Pup NOEL = 0.5 mg/kg (F1
survival was drastically decreased throughout lactation at 100
mg/kg. Clinical effects were increased in pups at 100 mg/kg,
primarily tonoclonic convulsions, no milk in stomach, entire litter
loss and death. Pup weights were statistically
significantly decreased at 100 mg/kg. Cross-fostering data
indicate the proximate toxicant (either XDE-007 and/or its metabolites)
led to decreased pup survival through the maternal milk and not
through exposure in utero. Possible adverse effect indicated:
excessive neonatal/pup mortality and increased
tonoclonic convulsions in pups (17/24 litters) at 100 mg/kg.
These data are supplemental. M. Silva, 7/20/04
-- “XDE-007: Two-Generation Dietary
Reproduction Toxicity Study in CD Rats,” (Carney,
E.W., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology &
Environmental Research and Consulting, The Dow Chemical Company,
Midland, MI; 3/22/04). XDE -007 (97.9% pure) was fed in diet to
Crl:CD (SD) IGS BR rats (30/sex/dose/generation) at 0, 0.5, 5
or 25 mg/kg/day continuously from pre-mating of parental generation
1 (P1) through weaning of offspring through 2 generations (2 matings
for P2 generation) to F2b weaning. Parental Systemic NOEL = 5.0
mg/kg/day (There were increased absolute
and relative liver weights in both sexes of P1 and P2 adults at
25 mg/kg and in P1 female liver weights at 5.0 mg/kg. P1
male relative kidney weights, P2 female absolute kidney weights,
P2 male relative spleen and thyroid weights and P2 female absolute
and relative adrenal weights were increased at 25 mg/kg.) ...
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Endocrine:
Testicular (click
on for all fluorinated pesticides)
-- REPRODUCTION, RAT. “XDE-007: Two-Generation
Dietary Reproduction Toxicity Study in CD Rats,”
(Carney, E.W., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology
& Environmental Research and Consulting, The Dow Chemical
Company, Midland, MI; 3/22/04). XDE -007 (97.9% pure) was fed
in diet to Crl:CD (SD) IGS BR rats (30/sex/dose/generation) at
0, 0.5, 5 or 25 mg/kg/day continuously from pre-mating of parental
generation 1 (P1) through weaning of offspring through 2 generations
(2 matings for P2 generation) to F2b weaning.. ... Reproduction
and Fertility NOEL = 5.0 mg/kg/day (There
was an increased proportion of abnormal P2 sperm at 25 mg/kg.
Although not statistically significant, there were decreased mating
and fertility indices in both sexes of P2a and P2b adults at >
5.0 mg/kg.)... F2a male weanlings had statistically significantly
decreased body weights, relative brain and absolute spleen weights
at 25 mg/kg. Neonates at 25 mg/kg in both F1 and F2 generations
showed tonoclonic convulsions, as well as in 1 litter of F2a at
5.0 mg/kg. P2 females (1, 1, 5 and 7 at 0, 0.5, 5.0 and 25 mg/kg,
respectively) failed to litter, so the female is considered to
be an affected sex.) Possible adverse effects on reproduction,
fertility and pup survival, along with numerous other toxicologically
relevant effects. M. Silva, 6/18/04
-- “XDE-007: Two-year Dietary Chronic
Toxicity/Oncogenicity and Chronic Neurotoxicity Study in Fischer
344 Rats,” (Yano, B.L., Dryzga, M.D., Thomas, J.;
Toxicology & Environmental Research and Consulting, The Dow
Chemical Company, Midland, MI; Laboratory Project Study ID: 011168;
4.22.05). Noviflumuron ((N-[3,5-dichloro-2-fluoro-4(1,1,2,3,3,3-hexafluoropropoxy)phenyl]-N’-(2,6-difluorobenzoyl)urea;
XDE-007, 97.9% pure) was fed in diet to Fischer 344 rats (75/sex/dose)
for 90 days, 1 or 2 years at 0, 0.1, 1.0, 75 or 300 mg/kg/day.
Subchronic toxicity was assessed after 90 days of treatment on
10/sex/dose at 0 and 1.0 mg/kg/day doses only. At 1 year,10/sex/dose
(chronic toxicity group), and 5/sex/dose (chronic neurotoxicity
group) were necropsied. The remaining 50/sex/dose were necropsied
after 2 years (oncogenicity group). Chronic NOEL = 1.0 mg/kg/day
(At > 75 mg/kg/day there were decreased body weights and body
weight gains along with increases or decreases
in absolute and/or relative organ weights (liver, kidney,
brain, heart, adrenal, testes, spleen,
epididymides were affected) at 12
and/or 24 months. Skin/tail papules and pustules were observed
in males (300 mg/kg/day) and females (> 75 mg/kg/day) in the
second year. Females showed an increase in phthisis bulbi at 300
mg/kg/day. Prothrombin time in males and cholesterol levels in
females were increased at > 75 mg/kg/day at 24 months. ALP
activities were increased in both sexes at > 75 mg/kg/day at
24 months. Urine specific gravity was decreased (both sexes at
> 75 mg/kg/day) and urine volume was increased (M 300 mg/kg/day
and F > 75 mg/kg/day) at 24 months. Lung inflammation, hepatocytic
hypertrophy (both sexes > 75 mg/kg/day), mineralization of
renal pelvic epithelium (M > 75 mg/kg/day), epididymal
aspermia*
(M > 75 mg/kg/day), atrophy of
seminiferous tubules (300 mg/kg/day), tail hyperkeratosis
+/- inflammation (both sexes 300 mg/kg/day), tail tip necrosis
(M 300 mg/kg/day) and hyperplasia of renal pelvic epithelium (M
300 mg/kg/day) were observed at 24 months. Leukemia was decreased
in both sexes at > 75 mg/kg/day at 24 months.) Possible adverse
effect: Hepatocellular adenomas (benign, M 300 mg/kg/day), uterine
stromal polyps (F > 75 mg/kg/day), and liver foci (M > 75
mg/kg/day) were increased at 24 months. Acceptable. Silva, 8/19/05
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
* Definition: Aspermia
= the complete absence of semen.
Endocrine:
Uterine (click
on for all fluorinated pesticides)
-- “XDE-007: Two-year Dietary Chronic
Toxicity/Oncogenicity and Chronic Neurotoxicity Study in Fischer
344 Rats,” (Yano, B.L., Dryzga, M.D., Thomas, J.;
Toxicology & Environmental Research and Consulting, The Dow
Chemical Company, Midland, MI; Laboratory Project Study ID: 011168;
4.22.05). Noviflumuron ((N-[3,5-dichloro-2-fluoro-4(1,1,2,3,3,3-hexafluoropropoxy)phenyl]-N’-(2,6-difluorobenzoyl)urea;
XDE-007, 97.9% pure) was fed in diet to Fischer 344 rats (75/sex/dose)
for 90 days, 1 or 2 years at 0, 0.1, 1.0, 75 or 300 mg/kg/day.
Subchronic toxicity was assessed after 90 days of treatment on
10/sex/dose at 0 and 1.0 mg/kg/day doses only. At 1 year,10/sex/dose
(chronic toxicity group), and 5/sex/dose (chronic neurotoxicity
group) were necropsied. The remaining 50/sex/dose were necropsied
after 2 years (oncogenicity group). Chronic NOEL = 1.0 mg/kg/day
(At > 75 mg/kg/day there were decreased body weights and body
weight gains along with increases or decreases
in absolute and/or relative organ weights (liver, kidney,
brain, heart, adrenal, testes, spleen, epididymides
were affected) at 12 and/or 24 months. Skin/tail papules and pustules
were observed in males (300 mg/kg/day) and females (> 75 mg/kg/day)
in the second year. Females showed an increase in phthisis bulbi
at 300 mg/kg/day. Prothrombin time in males and cholesterol levels
in females were increased at > 75 mg/kg/day at 24 months. ALP
activities were increased in both sexes at > 75 mg/kg/day at
24 months. Urine specific gravity was decreased (both sexes at
> 75 mg/kg/day) and urine volume was increased (M 300 mg/kg/day
and F > 75 mg/kg/day) at 24 months. Lung inflammation, hepatocytic
hypertrophy (both sexes > 75 mg/kg/day), mineralization of
renal pelvic epithelium (M > 75 mg/kg/day), epididymal aspermia
(M > 75 mg/kg/day), atrophy of seminiferous tubules (300 mg/kg/day),
tail hyperkeratosis +/- inflammation (both sexes 300 mg/kg/day),
tail tip necrosis (M 300 mg/kg/day) and hyperplasia of renal pelvic
epithelium (M 300 mg/kg/day) were observed at 24 months. Leukemia
was decreased in both sexes at > 75 mg/kg/day at 24 months.)
Possible adverse effect: Hepatocellular adenomas (benign, M 300
mg/kg/day), uterine stromal polyps (F >
75 mg/kg/day), and liver foci (M > 75 mg/kg/day) were increased
at 24 months. Acceptable. Silva, 8/19/05
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Eye
(click
on for all fluorinated pesticides)
-- “XDE-007: Two-year Dietary Chronic
Toxicity/Oncogenicity and Chronic Neurotoxicity Study in Fischer
344 Rats,” (Yano, B.L., Dryzga, M.D., Thomas, J.;
Toxicology & Environmental Research and Consulting, The Dow
Chemical Company, Midland, MI; Laboratory Project Study ID: 011168;
4.22.05). Noviflumuron ((N-[3,5-dichloro-2-fluoro-4(1,1,2,3,3,3-hexafluoropropoxy)phenyl]-N’-(2,6-difluorobenzoyl)urea;
XDE-007, 97.9% pure) was fed in diet to Fischer 344 rats (75/sex/dose)
for 90 days, 1 or 2 years at 0, 0.1, 1.0, 75 or 300 mg/kg/day.
Subchronic toxicity was assessed after 90 days of treatment on
10/sex/dose at 0 and 1.0 mg/kg/day doses only. At 1 year,10/sex/dose
(chronic toxicity group), and 5/sex/dose (chronic neurotoxicity
group) were necropsied. The remaining 50/sex/dose were necropsied
after 2 years (oncogenicity group). Chronic NOEL = 1.0 mg/kg/day
(At > 75 mg/kg/day there were decreased body weights and body
weight gains along with increases or decreases
in absolute and/or relative organ weights
(liver, kidney, brain, heart, adrenal, testes, spleen, epididymides
were affected) at 12 and/or 24 months. Skin/tail papules and pustules
were observed in males (300 mg/kg/day) and females (> 75 mg/kg/day)
in the second year. Females showed an increase
in phthisis bulbi*
at 300 mg/kg/day...
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
*
Definition: Phthisis
bulbi is an end-stage ocular response
to severe ocular disease, inflammation, or insult. Clinically,
the globe will be soft and hypotonus with minimal or no vision
and loss of much of the normal architecture of the eye.
Histopathologically, the globe is small and shrunken with marked
thickening of the sclera, as well as disorganization and atrophy
of much of the intraocular contents. The intraocular contents
are markedly dirupted and difficult to recognize. Commonly,
the retinal pigment epithelium may undergo a metaplasia leading
to intraocular ossification or bone formation in the end-stage
of phthisis bulbi.
Ref: University of Utah. John A. Moran
Eye Center.
http://insight.med.utah.edu/opatharch/uvea/phthisis_bubli.htm
Kidney
(click
on for all fluorinated pesticides)
-- “XDE-007: Two-year Dietary Chronic
Toxicity/Oncogenicity and Chronic Neurotoxicity Study in Fischer
344 Rats,” (Yano, B.L., Dryzga, M.D., Thomas, J.;
Toxicology & Environmental Research and Consulting, The Dow
Chemical Company, Midland, MI; Laboratory Project Study ID: 011168;
4.22.05). Noviflumuron ((N-[3,5-dichloro-2-fluoro-4(1,1,2,3,3,3-hexafluoropropoxy)phenyl]-N’-(2,6-difluorobenzoyl)urea;
XDE-007, 97.9% pure) was fed in diet to Fischer 344 rats (75/sex/dose)
for 90 days, 1 or 2 years at 0, 0.1, 1.0, 75 or 300 mg/kg/day.
Subchronic toxicity was assessed after 90 days of treatment on
10/sex/dose at 0 and 1.0 mg/kg/day doses only. At 1 year,10/sex/dose
(chronic toxicity group), and 5/sex/dose (chronic neurotoxicity
group) were necropsied. The remaining 50/sex/dose were necropsied
after 2 years (oncogenicity group). Chronic NOEL = 1.0 mg/kg/day
(At > 75 mg/kg/day there were decreased body weights and body
weight gains along with increases or decreases
in absolute and/or relative organ weights
(liver, kidney, brain, heart, adrenal,
testes, spleen, epididymides were affected) at 12 and/or 24 months.
Skin/tail papules and pustules were observed in males (300 mg/kg/day)
and females (> 75 mg/kg/day) in the second year. Females showed
an increase in phthisis bulbi at 300 mg/kg/day. Prothrombin time
in males and cholesterol levels in females were increased at >
75 mg/kg/day at 24 months. ALP activities were increased in both
sexes at > 75 mg/kg/day at 24 months. Urine specific gravity
was decreased (both sexes at > 75 mg/kg/day) and urine volume
was increased (M 300 mg/kg/day and F > 75 mg/kg/day) at 24
months. Lung inflammation, hepatocytic hypertrophy (both sexes
> 75 mg/kg/day), mineralization of renal
pelvic epithelium (M > 75 mg/kg/day), epididymal aspermia*
(M > 75 mg/kg/day), atrophy of seminiferous tubules
(300 mg/kg/day), tail hyperkeratosis +/- inflammation (both sexes
300 mg/kg/day), tail tip necrosis (M 300 mg/kg/day) and hyperplasia
of renal pelvic epithelium (M 300 mg/kg/day) were observed
at 24 months. Leukemia was decreased in both sexes at > 75
mg/kg/day at 24 months.) Possible adverse effect: Hepatocellular
adenomas (benign, M 300 mg/kg/day), uterine stromal polyps (F
> 75 mg/kg/day), and liver foci (M > 75 mg/kg/day) were
increased at 24 months. Acceptable. Silva, 8/19/05
-- “XDE-007: Two-Generation Dietary
Reproduction Toxicity Study in CD Rats,” (Carney,
E.W., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology &
Environmental Research and Consulting, The Dow Chemical Company,
Midland, MI; 3/22/04). XDE -007 (97.9% pure) was fed in diet to
Crl:CD (SD) IGS BR rats (30/sex/dose/generation) at 0, 0.5, 5
or 25 mg/kg/day continuously from pre-mating of parental generation
1 (P1) through weaning of offspring through 2 generations (2 matings
for P2 generation) to F2b weaning. Parental Systemic NOEL = 5.0
mg/kg/day (There were increased absolute
and relative liver weights in both sexes of P1 and P2 adults at
25 mg/kg and in P1 female liver weights at 5.0 mg/kg. P1
male relative kidney weights, P2 female absolute kidney weights,
P2 male relative spleen and thyroid weights and P2 female absolute
and relative adrenal weights were increased at 25 mg/kg.) ...
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Liver
(click on for all fluorinated
pesticides)
“XDE-007: 18-Month Oncogenicity Study
in CD-1 Mice,” (Johnson, K.A.; Toxicology & Environmental
Research and Consulting, The Dow Chemical Company, Midland, MI;
4/25/05). XDE-007 technical (N-[3,5-dichloro-2-fluoro-4-(1,1,2,3,3,3hexafluoropropoxy)phenyl]-N’-(2,6-difluorobenzoyl)urea),
97.9% pure) was fed in diet to CD-1 mice (50/sex/dose) at 0, 0.5,
3 (males only), 30 and 100 (females only) mg/kg/day for up to
18 months. Systemic NOEL = 3 mg/kg/day (males) and 30 mg/kg/day
(females) There was an increase in mortality for females at 100
mg/kg/day (M: 56% vs 32%). There was an increased incidence in
tonoclonic convulsions (primarily in females) at the high dose.
There was an increase in relative and absolute liver weights in
females at > 30 mg/kg/day. Males had an absolute liver weight
increase at 30 mg/kg/day and a relative increase at > 3 mg/kg/day.
Both sexes showed an increase in all severities of liver hypertrophy
(hepatocytic, centrilobular/midzonal, diffuse) at 30 mg/kg/day
(male) and at 100 mg/kg/day (female)... Possible
adverse effect indicated: There was an increased incidence in
hepatocytic adenomas and in hepatocytic adenomas plus carcinomas
(slight in males, statistically significant in females) at 30
mg/kg/day (5, 7, 4, 12 of 50) and 100 mg/kg/day (F: 0, 1, 0, 4**
of 50 by Peto’s mortality adjusted statistics ).
Females showed an increased incidence in lung carcinomas (non-metastatic)
at 100 mg/kg/day (0, 0, 1, 2** by Peto’s statistics).
-- “XDE-007: Two-Generation Dietary
Reproduction Toxicity Study in CD Rats,” (Carney,
E.W., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology &
Environmental Research and Consulting, The Dow Chemical Company,
Midland, MI; 3/22/04). XDE -007 (97.9% pure) was fed in diet to
Crl:CD (SD) IGS BR rats (30/sex/dose/generation) at 0, 0.5, 5
or 25 mg/kg/day continuously from pre-mating of parental generation
1 (P1) through weaning of offspring through 2 generations (2 matings
for P2 generation) to F2b weaning. Parental Systemic NOEL = 5.0
mg/kg/day (There were increased absolute
and relative liver weights in both sexes of P1 and P2 adults at
25 mg/kg and in P1 female liver weights at 5.0 mg/kg...
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
-- (Oral) 008; 186500; "XR-007: 4-Week
Dietary Toxicity Study in Fischer Rats" (Lick, S.J.
et al., Health & Environmental Research Laboratories, The Dow
Chemical Company, Midland, MI, Laboratory Project Study
ID 971106, 10/9/97). XR-007 (Lot # DECO-615-112, purity = 99.6%)
was admixed to the feed and fed to 5 Fischer 344 rats per sex
per dose at dose levels of 0, 1, 10, 100, 500, or 1000 mg/kg/day
(0, 1.0, 10.4, 101.4, 512.6, 1029.1 mg/kg/day, respectively for
males and 0, 1.1, 10.9, 105.1, 520.6, 1055.6 mg/kg/day, respectively
for females) for 4 weeks... A treatment-related increase in mean
relative liver weight was observed
in both sexes at 500 and 1000 mg/kg/day. Microscopic examination
revealed treatment-related hepatocellular
hypertrophy (centrilobular) in males at 1000 mg/kg/day
and in females at 500 and 1000 mg/kg/day. No adverse effects.
NOEL (M) = 101.4 mg/kg/day and NOEL (F) = 105.1 mg/kg/day (based
on an increase in mean relative liver weight
and hepatocellular hypertrophy). Supplemental (because
only 5 animals per sex per dose were used, the animals were treated
for only 4 weeks, no ophthalmological examinations were conducted,
and no analysis of the dosing material was conducted). (Corlett,
10/3/02)
-- 007; 186499; "XDE-007: 28-Day Dietary
Toxicity Study in CD-1 Mice" (Yano, B.L. and Day,
S.J., Toxicology & Environmental Research and Consulting, The
Dow Chemical Company, Midland,
MI, Laboratory Project Study ID 001248, 6/12/01). XDE-007 (Lot,
Reference No. F0031-148, TSN102332, purity = 98.4%) was admixed
to the feed and fed to 5 CD-1 mice per sex per dose at dose levels
of 0, 10, 100, 500, or 1000 mg/kg/day (0, 10.8, 110, 538, 1060
mg/kg/day, respectively for males and 0, 11.2, 113, 504, 1140
mg/kg/day, respectively for females) for 28 days. No mortalities
occurred. No treatment-related clinical signs were observed. Treatment-related
increases in mean platelet level and mean
cholesterol level were observed in both sexes at 100, 500,
and 1000 mg/kg/day. A treatment-related increase in mean relative
liver weight was observed at in males
at 100, 500, and 1000 mg/kg/day and in females at 500 and 1000
mg/kg/day. Microscopic examination revealed treatment-related
hepatocellular hypertrophy with altered tinctorial properties
(centrilobular/midzonal to panlobular) in males at 500 and 1000
mg/kg/day and very slight vacuolation (consistent with fatty change)
of the periportal hepatocytes in
males at 500 and 1000 mg/kg/day and in females at 1000 mg/kg/day.
No adverse effects. NOEL (M) = 10.8 mg/kg/day and NOEL (F) = 11.2
mg/kg/day (based on increases in mean platelet
and mean cholesterol levels). Supplemental (because only 5 animals
per sex per dose were used and because the animals were
treated for only 28 days). (Corlett, 9/30/02)
Ref: September 26, 2002. Summary of Toxicology
Data for Noviflumuron ((XDE-007) or N-(((3,5-dichloro-2-fluoro-4-(1,1,2,3,3,3-hexafluoropropoxy)phenyl)amino)carbonyl)-2,6-
diflurobenzamide. California EPA, Department of Pesticide Regulation,
Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Noviflumuron.CA.EPA.2002.pdf
Lung
(click
on for all fluorinated pesticides)
“XDE-007: 18-Month Oncogenicity Study in CD-1 Mice,”
(Johnson, K.A.; Toxicology & Environmental Research and Consulting,
The Dow Chemical Company, Midland, MI; 4/25/05). XDE-007 technical
(N-[3,5-dichloro-2-fluoro-4-(1,1,2,3,3,3hexafluoropropoxy)phenyl]-N’-(2,6-difluorobenzoyl)urea),
97.9% pure) was fed in diet to CD-1 mice (50/sex/dose) at 0, 0.5,
3 (males only), 30 and 100 (females only) mg/kg/day for up to
18 months. Systemic NOEL = 3 mg/kg/day (males) and 30 mg/kg/day
(females)... There was an increased incidence
in evidence of inflammation in lung in both sexes at the high
dose... Females showed an increased incidence in lung carcinomas
(non-metastatic) at 100 mg/kg/day (0, 0, 1, 2** by Peto’s
statistics).
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Reproductive
(click
on for all fluorinated pesticides)
-- “XDE-007: One-Generation Dietary
Reproduction Toxicity Study With CrossFostering in CD Rats,”
(Marty, M.S., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology
& Environmental Research and Consulting, The Dow Chemical
Company, Midland, MI; Laboratory Project Study ID: 011221; 3/23/04).
XDE -007 (97.9% pure) was fed in diet to Crl:CD (SD) IGS BR rats
(30/sex/dose) at 0, 0.5, 5 and 100 mg/kg/day continuously from
pre-mating (10 weeks) of parental generation, through breeding
(2 weeks), gestation (3 weeks) and lactation through weaning of
F1 offspring. Although designed as a 2 generation
reproduction study, it was terminated after 1 generation due to
excessive F1 pup mortality at 100 mg/kg/day. Subsequently
a Cross-fostering Study was performed to determine whether the
decreased survival in pups from XDE-007 treated P1 rats resulted
from in utero or lactational exposure. Parental Systemic NOEL
= 0.5 mg/kg/day (Tonoclonic convulsions were observed in 2/30
males and 1/24 females at 100 mg/kg/day. Males had statistically
significantly decreased food consumption from day 8 until termination
at 100 mg/kg. Females had statistically significantly decreased
food consumption during lactation. Male
P1 premating body weights were statistically significantly decreased
at 100 mg/kg/day. Mean gestational body
weight gain in females was statistically significantly decreased
GD 0 - 7 (but not GD 0 - 21) and lactation days 7 - 13 at 100
mg/kg/day.) Reproduction and Fertility NOEL = 0.5 mg/kg/day (P1
female gestation survival was decreased and gestation length was
increased at 100 mg/kg. Pup NOEL = 0.5 mg/kg (F1
survival was drastically decreased throughout lactation at 100
mg/kg. Clinical effects were increased in pups at 100 mg/kg,
primarily tonoclonic convulsions, no milk in stomach, entire litter
loss and death. Pup weights were statistically
significantly decreased at 100 mg/kg. Cross-fostering data
indicate the proximate toxicant (either XDE-007 and/or its metabolites)
led to decreased pup survival through the maternal milk and not
through exposure in utero. Possible adverse effect indicated:
excessive neonatal/pup mortality and increased
tonoclonic convulsions in pups (17/24 litters) at 100 mg/kg.
These data are supplemental. M. Silva, 7/20/04
-- “XDE-007: Two-Generation Dietary
Reproduction Toxicity Study in CD Rats,” (Carney,
E.W., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology &
Environmental Research and Consulting, The Dow Chemical Company,
Midland, MI; 3/22/04). XDE -007 (97.9% pure) was fed in diet to
Crl:CD (SD) IGS BR rats (30/sex/dose/generation) at 0, 0.5, 5
or 25 mg/kg/day continuously from pre-mating of parental generation
1 (P1) through weaning of offspring through 2 generations (2 matings
for P2 generation) to F2b weaning. Parental Systemic NOEL = 5.0
mg/kg/day (There were increased absolute
and relative liver weights in both sexes of P1 and P2 adults at
25 mg/kg and in P1 female liver weights at 5.0 mg/kg. P1
male relative kidney weights, P2 female absolute kidney weights,
P2 male relative spleen and thyroid weights and P2 female absolute
and relative adrenal weights were increased at 25 mg/kg.) Reproduction
and Fertility NOEL = 5.0 mg/kg/day (There
was an increased proportion of abnormal P2 sperm at 25 mg/kg.
Although not statistically significant, there were decreased mating
and fertility indices in both sexes of P2a and P2b adults at >
5.0 mg/kg.) Pup NOEL = 5.0 mg/kg (There was a decrease
in F1 survival (not statistically significant) on lactation days
14 and 21 at 25 mg/kg. F2a pups had statistically
significantly decreased survival of lactation days 7, 14 and 21at
25 mg/kg. Mean F2a litter size was statistically significantly
decreased on lactation days 14 and 21 at 25 mg/kg. Mean
F1 pup weights were statistically significantly decreased at 25
mg/kg on lactation days 1, 14 and 21. Mean
F2a male pup weights were statistically significantly decreased
on lactation days 1 and 21 and F2a female pup weights were
decreased on lactation day 21 at 25 mg/kg.
F2a male weanlings had statistically significantly decreased body
weights, relative brain and absolute spleen weights at 25 mg/kg.
Neonates at 25 mg/kg in both F1 and F2 generations showed tonoclonic
convulsions, as well as in 1 litter of F2a at 5.0 mg/kg.
P2 females (1, 1, 5 and 7 at 0, 0.5, 5.0 and 25 mg/kg, respectively)
failed to litter, so the female is considered to be an affected
sex.) Possible adverse effects on reproduction, fertility and
pup survival, along with numerous other toxicologically relevant
effects. M. Silva, 6/18/04
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Spleen
(click
on for all fluorinated pesticides)
-- REPRODUCTION, RAT. “XDE-007: Two-Generation
Dietary Reproduction Toxicity Study in CD Rats,”
(Carney, E.W., Zablotny, C.L., Liberacki, A.B., Yano, B.L.;Toxicology
& Environmental Research and Consulting, The Dow Chemical
Company, Midland, MI; 3/22/04). XDE -007 (97.9% pure) was fed
in diet to Crl:CD (SD) IGS BR rats (30/sex/dose/generation) at
0, 0.5, 5 or 25 mg/kg/day continuously from pre-mating of parental
generation 1 (P1) through weaning of offspring through 2 generations
(2 matings for P2 generation) to F2b weaning. . ... . F2a
male weanlings had statistically significantly decreased body
weights, relative brain and absolute
spleen weights at 25 mg/kg.
Neonates at 25 mg/kg in both F1 and F2 generations showed
tonoclonic convulsions, as well as in 1 litter of F2a at 5.0 mg/kg.
P2 females (1, 1, 5 and 7 at 0, 0.5, 5.0 and 25 mg/kg, respectively)
failed to litter, so the female is considered
to be an affected sex.) Possible adverse effects on reproduction,
fertility and pup survival, along with numerous other toxicologically
relevant effects. M. Silva, 6/18/04
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Stomach
(click
on for all fluorinated pesticides)
-- Chronic Study: “XDE-007: One-Year
Dietary Toxicity Study in Beagle Dogs,” (Stebbins,
K.E., Day, S.J., Thomas, J.; Toxicology & Environmental Research
and Consulting, The Dow Chemical Company, Midland, MI; Laboratory
Project Study ID #: 142640; 3/5/04). XDE-007 (97.9% pure) was
fed in diet to Beagle dogs (4/sex/dose) for 1 year at 0, 0.003,
0.03 or 0.225% (equivalent to 0, 0.74, 9.3 and 69 mg/kg/day -
Males and 0, 0.94, 8.7 and 70 mg/kg/day - females). NOEL = 0.003%
(0.74 mg/kg/day - Males; 0.94 mg/kg/day - Females) (Mean corpuscular
volume in males (compared with controls at each time interval)
and reticulocytes in both sexes were increased at 0.225% throughout
the study. Both sexes at > 0.225% had statistically significantly
increased mean platelet count. There was a significant increase
in ALP at 0.225% in females at 3 and 12 months. There was a statistically
significant increase in absolute adrenal weights (analyzed across
both sexes) at 0.225% without histological findings. There was
an increased incidence in bone marrow erythroid hyperplasia (erythroid
cell) at > 0.3% XDE-007 (males, 0, 0, 4, 4 --each dose level)
and at 0.225% (females: 0, 0, 0, 1 --each dose level).
There was a dose-related increase in severity of stomach mucosal
lymphoid hyperplasia in the fundus and pylorus in both sexes at
> 0.3%.) No adverse effect. Acceptable. (M. Silva, 6/21/04).
Ref:
August 2005 - Summary of toxicological data. California EPA, Department
of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2005.pdf
Teratogen
(click
on for all fluorinated pesticides)
-- 52905-009 186501,
"XR-007: Whole Embryo Culture Teratogenicity
Screen", (E. W. Carney, Health and Environmental Research
Laboratories, The Dow Chemical Company,
Midland, MI, Report # 971083, 31 July 1997). Seven non-pregnant
female rats (serum donors) received 1000 mg/kg/day of the test
article (XR-007, 98%) in 0.5% Methocel by gavage for 3 consecutive
days. Four hours after the last dose, rats were exsanguinated
and their blood centrifuged to obtain serum. Six control rats
were similarly treated with vehicle and bled...
Statistically significant increases in crown-rump length and somite
number for treated embryos were reportedly due to a lower
than usual growth rate in control embryos. Morphological
abnormality was limited to an abnormal curvature of the anterior
neural tube which distorted the head in one treated embryo
(8.3%). No historical control data. This is supplemental information.
(Green and Gee, 10/3/02).
Ref:
September 26, 2002. Summary of Toxicology Data for Noviflumuron
((XDE-007) or N-(((3,5-dichloro-2-fluoro-4-(1,1,2,3,3,3-hexafluoropropoxy)phenyl)amino)carbonyl)-2,6-
diflurobenzamide. California EPA, Department of Pesticide Regulation,
Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Noviflumuron.CA.EPA.2002.pdf
[Note
from FAN- some definitions:
"Predominately anterior neural tube
defects, occuring early in gestation. The anterior neural
tube fails to close properly, or does not divide into two hemispheres.
The generic name for this category of malformations is arrhinencephaly.
Ref: Developmental and Perinatal Disorders of the CNS. Weill
Medical College of Cornell University. http://edcenter.med.cornell.edu/CUMC_PathNotes/Neuropathology/Neuropath_II/dev2.html
-- "... trisomy 13 is closely linked to midline malformations,
including arrhinencephaly,
agenesis of the corpus callosum, and holoprosencephaly, and
is the single most common chromosomal defect underlying holoprosencephaly."
- http://128.100.71.82/neurosurgery/developmental.html
-- "Trisomy 13 Syndrome is
a genetic disorder with onset before birth. It occurs approximately
1/5000 live births. Infants affected with Trisomy 13 tend
to be small at birth. Spells of interrupted breathing (apnea)
in early infancy are frequent, and mental retardation is usually
severe. Many affected children appear to be deaf. A moderately
small head (microcephaly) with sloping forehead, wide joints
and openings between parietal bones of the head are present.
Gross anatomic defects of the brain, especially failure of the
forebrain to divide properly (holoprosencephaly) are common.
A hernial protrusion of the cord and its meninges through a
defect in the vertebral canal (myelomeningocele) is found in
almost 50% of cases. The entire eye is usually small (microphthalmia),
and a defect of the iris tissue (coloboma), and faulty development
of the retina (retinal dysplasia) occur frequently. The supraorbital
ridges are shallow and palapebral fissures are usually slanted.
Cleft lip, cleft palate, or both are present in most cases.
The ears are abnormally shaped and unusually low-set."
-
http://www.trisomy.org/html/trisomy_13_facts.htm
|
Environmental (click
on for all fluorinated pesticides)
The
data indicate that noviflumuron is relatively non-toxic
to vertebrate animals, birds, moderately toxic to fish
and highly toxic to freshwater invertebrates.
The proposed label does bear precautionary statements
regarding the high toxicity to freshwater invertebrates.
The environmental fate data indicates noviflumuron is
strongly bound to soil and has low water solubility indicating
a low potential for soil leaching. The proposed use for
noviflumuron as a self contained bait to control termites
around structures, fence posts, utility poles and landscape
plantings is not expected to pose a treat to wildlife.
Ref:
2003.
Noviflumuron.
California Department of Pesticide Regulation. Public
Report 2003-5.
http://www.fluorideaction.org/pesticides/noviflumuron.ca.epa.2003.pdf
|
|

