|
Return
to
Index Page
Abstracts
US
Food Tolerances
Activity:
Herbicide
(pyridazinone)
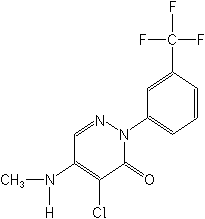
Structure:
Adverse
Effects:
Blood
Body Weight Decrease
Bone
Cancer: Possible Human Carcinogen - LIVER
Cholesterol
Endocrine: Ovary
Endocrine: Thyroid
Endocrine: Uterus
Kidney
Liver
Spleen
Environmental
•
As of February 12, 2005, this herbicide is permitted in
or on 59 food commodities
in the United States - click
here to food commodities
•
Norflurazon - which was on a low-priority sampling list
in California because it was not anticipated to pollute
groundwater - was found in groundwater by Florida which
is more vigilant about water quality because the state is
experiencing a water pollution crisis. California found
norflurazon - the third most popular
herbicide used on the state's roadsides - in 9.5%
of the wells in 1997, the first time samples were taken.
Ref: Pathways
of Exposure. Californians for
Alternatives to Toxics.
|
Blood
(click
on for all fluorinated pesticides)
--Chronic Toxicity
and Carcinogenicity. In a 6-month toxicity study, norflurazon
technical was administered in the diet to male and female beagle
dogs (4/sex/group) at dose levels of 0, 50, 150, or 450 ppm (0,
1.53, 5.02, and 14.27 mg/kg for males; 0, 1.58, 4.77, and 17.75
mg/kg for females). At the 150 ppm dose
level, liver weight was increased by 38% in male dogs and by 23%
in female dogs. Thyroid weight was increased by 33% in male dogs
and 37% in female dogs at this dose level. Also noted at the 150
ppm dose level were increases in cholesterol in both sexes (23-40%
in males, 6-34% in females), a decrease
in SGPT (36-38% in males, 13-20% in females) and
SGOT (4-23% in males, 13-23% in females). At the 450 ppm
dose level, similar changes were observed in male and female dogs,
with the additional observation of a decrease
in red cell count in female dogs (79-92% of control). The
systemic NOEL was determined to be 50 ppm (1.53 mg/kg/day [males];
1.58 mg/kg/day [females] )...
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/pesticides/norflurazon.red.epa.pdf
Body
Weight Decrease
(click
on for all fluorinated pesticides)
--
Developmental and Reproductive Toxicity In a developmental toxicity
(teratology) study, rats of the Sprague-Dawley strain received
either 0, 100, 200, or 400 mg/kg/day norflurazon technical by
oral gavage on gestation days 6 through 15 inclusive. The maternal
toxicity NOEL was determined to be < 100 mg/kg/day, and the maternal
toxicity LEL was determined to be < 100 mg/kg/day, based on reductions
in body weight gain for the period of dosing and for the
dosing plus post-dosing period. The developmental toxicity NOEL
was determined to be > 400 mg/kg/day, and the developmental toxicity
LEL was determined to be > 400 mg/kg/day...
-- The studies examining developmental and reproductive toxicity
of norflurazon were considered by the Health Effects Division
RfD/Peer Review Committee in a meeting held March 16, 1995. Overall,
the committee concluded that treatment with norflurazon was associated
with reproductive toxicity in the form of increased pup death,
increased stillborn pups, and increased pup deaths between days
5-14 of lactation at a dose of 1500 ppm. Developmental toxicity
was not evident in treated rats, but developmental toxicity in
rabbits was evident at a dose of 60 mg/kg/day in the form of decreased
mean fetal weight, slight delays in ossification of the
skull and limbs, and an increase in the incidence of 13th ribs.
These developmental effects occurred at a dose that was maternally
toxic.
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/pesticides/norflurazon.red.epa.pdf
Bone
(click
on for all fluorinated pesticides)
For females (13-50
years) population subgroup, the acute No Observed Adverse Effects
Level (NOAEL) of 10 mg/kg/day was established based on increased
incidence in skeletal variations
observed in the developmental toxicity study in rabbits at the
Lowest Observed Adverse Effect Level (LOAEL) of 30 mg/kg/day...
The FQPA safety factor to account for the enhanced sensitivity
of infants and children be reduced to 3X based on increased incidence
in skeletal variations observed in
the developmental toxicity study in rabbits. This determination
is based on the following: the toxicity database is adequate;
the development toxicity studies in the rat and rabbit are acceptable
as an multi-generation study; a developmental neurotoxic study
is not required because there is no indication of increased susceptibility
in young rats to norflurazon exposure; and there are currently
no residential uses for norflurazon. The FQPA safety factor is
applicable to only females 13-50 population subgroup for acute
dietary risk assessment.
Ref: US EPA May 31, 2002. Tolerance Reassessment
Progress and Risk Management Decision.
http://www.fluoridealert.org/pesticides/norflurazon.tred.may31.2002.pdf
A developmental toxicity
study was conducted in New Zealand White rabbits, which received
either 0, 10, 30, or 60 mg/kg/day norflurazon technical by oral
gavage on gestation days 7 through 19 inclusive... The developmental
toxicity NOEL was determined to be 30 mg/kg/day, and the developmental
toxicity LEL was determined to be 60 mg/kg/day, based on
statistically significant increases in the fetal incidence of
incompletely ossified frontal bones, 16
caudal vertebrae, unossified first metacarpal, unossified middle
phalanx of the fifth digit of the forelimb, and unossified proximal
epiphysis of the tibia at the 60 mg/kg/day dose level.
Ref: US EPA Reregistration Eligibility Decision:
Norflurazon. http://www.fluoridealert.org/pesticides/norflurazon.red.epa.pdf
-- A nine-month oral
toxicity study (MRID 00091056) was conducted using the F generation
of rats from a two-year carcinogenicity study (MRID 00082019).
In this study, rats were given technical norflurazon in the diet
at dose levels of 0, 125, 250, or 500 ppm (0, 6.25, 12.5, and
25 mg/kg/day) for 39 weeks. There were no significant effects
of norflurazon on survival, body weight, body weight gain, food
consumption, food efficiency, hematology, or clinical chemistry
in male or female rats at any dose level tested. At 500 ppm,
liver weight was increased by 14% and 23% in male and female
rats respectively, at 39 weeks. Kidney weight was not significantly
affected, but the incidence of hyaline pigment deposition (many
tubules) in male rats and hyaline pigment deposition (few tubules)
in female rats was increased, as was the incidence of
medullary congestion in high dose female rats... (guideline:
non-guideline study; classified as core supplementary; MRID 00091056).
-- Developmental and Reproductive Toxicity In a developmental
toxicity (teratology) study, rats of the Sprague-Dawley strain
received either 0, 100, 200, or 400 mg/kg/day norflurazon technical
by oral gavage on gestation days 6 through 15 inclusive. The maternal
toxicity NOEL was determined to be < 100 mg/kg/day, and the maternal
toxicity LEL was determined to be < 100 mg/kg/day, based on reductions
in body weight gain for the period
of dosing and for the dosing plus post-dosing period. The developmental
toxicity NOEL was determined to be > 400 mg/kg/day, and the developmental
toxicity LEL was determined to be > 400 mg/kg/day. Developmental
toxicity was suggested at the 400 mg/kg/day dose level in the
form of an increase in bipartite thoracic
vertebrae (10th-13th) and an increase in rudimentary 14th ribs.
However, these increases were not statistically significant and
are believed to be secondary to maternal effects at the high dose
(guideline 83-3; MRID 00063621).
-- A developmental toxicity study was conducted in New Zealand
White rabbits, which received either 0, 10, 30, or 60 mg/kg/day
norflurazon technical by oral gavage on gestation days 7 through
19 inclusive. The maternal toxicity NOEL was determined to be
30 mg/kg/day, and the maternal toxicity LEL was determined to
be 60 mg/kg/day, based on decreased body weight gain
and clinical toxicity (abortion) at this dose level. The
developmental toxicity NOEL was determined to be 30 mg/kg/day,
and the developmental toxicity LEL was determined to be 60 mg/kg/day,
based on statistically significant increases
in the fetal incidence of incompletely ossified frontal bones,
16 caudal vertebrae, unossified first metacarpal, unossified middle
phalanx of the fifth digit of the forelimb, and unossified proximal
epiphysis of the tibia at the 60 mg/kg/day dose level.
A 12% decrease in mean fetal weight
was also observed at the 60 mg/kg/day dose level (guideline 83-3;
MRID 00131151 and 00131152).
-- The studies examining developmental and reproductive toxicity
of norflurazon were considered by the Health Effects Division
RfD/Peer Review Committee in a meeting held March 16, 1995. Overall,
the committee concluded that treatment with norflurazon was associated
with reproductive toxicity in the form of
increased pup death, increased stillborn pups, and increased pup
deaths between days 5-14 of lactation at a dose of 1500
ppm. Developmental toxicity was not evident in treated rats, but
developmental toxicity in rabbits was evident at a dose of 60
mg/kg/day in the form of decreased mean fetal weight, slight
delays in ossification of the skull and limbs, and an increase
in the incidence of 13th ribs. These developmental effects
occurred at a dose that was maternally toxic.
-- Toxic Endpoints of Concern Identified for Use in Risk Assessment.
The Health Effects Division's (HED) Less than Lifetime Committee
selected the following toxicology endpoints for use in risk assessment
for norflurazon (Toxicology Endpoint Selection Document, 4/21/95):
The endpoint selected for the acute dietary risk assessment is
a NOEL of 30 mg/kg/day. This is the developmental NOEL based on
increased skeletal variations observed
at the dose of 60 mg/kg/day in a rabbit developmental toxicity
study.
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/pesticides/norflurazon.red.epa.pdf
Cancer:
Probable Human Carcinogen - LIVER (click
on for all fluorinated pesticides)
Group
C -- Possible Human Carcinogen. Statistically
significant increase in comparison to controls in liver
adenomas & combined liver adenomas & carcinomas, as well
as the statistically significant positive trend for these hepatocellular
adenomas & combined adenomas & carcinomas; CD-1 mice (M).
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Group C--Possible Human Carcinogen.
Reviewed 11/ 2/ 90.
Ref: List of Chemicals Evaluated
for Carcinogenic Potential. Science Information Management Branch,
Health Effects Division, Office of Pesticide Programs, U. S. Environmental
Protection Agency. March 15, 2002.
http://www.biomuncie.org/chemicals_evaluated_for_carcinog.htm
As
a result of the July 18, 1990 meeting of the OPP/Health Effects
Division Carcinogenicity Peer Review Committee, norflurazon was
classified as a non quantifiable Group C - possible
human carcinogen - based upon statistically significant
pair-wise comparisons of the incidence of liver
adenomas and combined liver adenomas/ carcinomas as well as statistically
positive trends for these lesions in male CD-1 mice receiving
218.8 mg/kg/day norflurazon technical in the diet for up to 104
weeks (MRID 00111649).
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/pesticides/norflurazon.red.epa.pdf
Cholesterol
(click
on for all fluorinated pesticides)
A 90-day rat feeding
study at nominal dosages of 0, 12.5, 25.0 and 125.0 mg/kg/day...
at the mid-dose level there were increases
of cholesterol in both sexes (23
to 40 percent in males, 6 to 34 percent in females), a
decrease in SGPT (36 to 38 percent in males, 13 to 20 percent
in females) and SGOT (4 to 23 percent in males, 13 to 23 percent
in females). At the highest level, similar changes were observed
in male and female dogs, with the additional observation of a
decrease in red cell count in female dogs (79 to 92 percent of
control). The systemic NOEL was determined to be 1.53 mg/kg/day
for males and 1.58 mg/kg/day for females. The systemic LEL was
determined to be 5.02 mg/kg/day for males and 4.77 mg/kg/day for
females, based on increased absolute and relative liver weight
and increased cholesterol in both sexes.
Ref: Federal Register: July 29, 1996 [Page
39347-39351]. Norflurazon; Pesticide Tolerance. Final Rule.
http://www.fluoridealert.org/pesticides/Norflurazon.FR.July.29.1996.htm
The chronic NOAEL of
1.5 mg/kg/day was established based on increased absolute and
relative liver weight and increased cholesterol
in both sexes in a 6-month feeding study in dogs at the LOAEL
of 4.77 mg/kg/day... The acute and chronic dietary exposure analyses
are based on the Dietary Exposure Evaluation Model (DEEM TM .)
The acute dietary exposure estimates used the entire distribution
of single day food consumption. The chronic dietary exposure estimates
the three day average consumption for each population subgroup.
The DEEM TM analysis was conducted using consumption data from
the USDA 1989-92 Continuing Surveys for Food Intake by Individuals
(CSFII).
Ref: US EPA May 31, 2002. Tolerance Reassessment
Progress and Risk Management Decision.
http://www.fluoridealert.org/pesticides/Norflurazon.TRED.May31.2002.pdf
Endocrine:
Ovary
(click
on for all fluorinated pesticides)
-- A two-year carcinogenicity
study was conducted in male and female CD-1 HaM/ICR Swiss mice
in which 125 mice/sex/dose were administered technical norflurazon
in the diet at dose levels of 0, 85, 340, or 1360 ppm (0, 12.8,
58.7, or 218.8 mg/kg/day) for 100-104 weeks...
Increased incidences of enlarged spleen, nephritis, swollen/enlarged
liver, and nodular enlargement of the liver were observed in high
dose male mice, while increased incidences of pyelonephritis,
enlarged liver, and
cystic ovaries were observed in high dose female mice.
Carcinogenic potential was evidenced by an increased incidence
of hepatic adenoma and combined adenoma/carcinoma
in high dose male mice. The systemic NOEL was determined
to be 12.8 mg/kg/day (85 ppm) for male mice, and 58.7 mg/kg/day
(340 ppm) for female mice. The systemic LEL was determined to
be 58.7 mg/kg/day (340 ppm) for male mice, based on the increased
incidence of enlarged spleen, increased
absolute and relative liver weight, and increased incidence of
nephritis. The systemic LEL was determined to be 218.8
mg/kg/day (1360 ppm) for female mice, based on the increased incidence
of enlarged liver and cystic
ovaries, the
increased absolute and relative liver weight,
and the increased incidence of pyelonephritis (guideline §83-2;
MRID 00111649).
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/PESTICIDES/Norflurazon.RED.EPA.pdf
-- A nine-month oral
toxicity study (MRID 00091056) was conducted using the F generation
of rats from a two-year carcinogenicity study (MRID 00082019).
In this study, rats were given technical norflurazon in the diet
at dose levels of 0, 125, 250, or 500 ppm (0, 6.25, 12.5, and
25 mg/kg/day) for 39 weeks... At 500 ppm...
Thyroid weight was decreased in males by approximately 30% vs
control,
while thyroid weight was increased in females between 11-47% vs
control. Gonadal
weight (left gonad) was increased in female rats by 30%.
At 250
ppm, thyroid weight was decreased by 30% in male rats, but was
increased by 11-29% in female rats. However, there were no reported
microscopic alterations in this organ at any dose level tested. Gonadal
weight in female rats was increased by 17% vs. control.
The systemic NOEL was determined to be 250 ppm (12.5 mg/kg/day)
in both sexes. The systemic LEL was determined to be 500 ppm (25
mg/kg/day) in both sexes, based on the dose-related increase in
liver weight in male and female rats
at 39 weeks, the increase in gonad weight
of females, and the microscopic changes observed in
kidneys of
both sexes. Although
dramatic effects on thyroid weight were
observed at 250 ppm in both sexes, there were no data indicating
any alteration in histology of this organ. Thus, the weight change,
while indicative of an effect of norflurazon, is not supported
as a toxic effect based on available data (guideline: non-guideline
study; classified as core supplementary; MRID 00091056).
Ref:
US EPA REREGISTRATION ELIGIBILITY DECISION NORFLURAZON. LIST A.
CASE 0229.
http://www.fluoridealert.org/PESTICIDES/Norflurazon.RED.EPA.pdf
Endocrine:
Thyroid
(click
on for all fluorinated pesticides)
Congestion of the liver,
hepatocyte swelling and increased liver weights, and increase
in colloid vacuole in the thyroid
were observed in dogs fed 450 ppm (10.25 mg/kg/day) norflurazon
for 6 months. The NOEL was 150 ppm (3.75 mg/kg/day). An oral RfD
of 0.04 mg/ kg/day has been determined. Increased relative liver
weight and hypertrophy of the thyroid
with depletion of colloid were seen in rats fed 2,500 ppm (125
mg/kg/day) norflurazon for 90 days. The NOEL was 500 ppm (25 mg/kg/day).
Hepatic hyperplasia and hypertrophy and increased relative liver
weight were noted in a 28-day feeding study in rats. The LOEL
was 1,000 ppm (50 mg/kg/day) and the NOEL was 500 ppm (25 mg/kg/
day). Increased relative liver weight and diffuse and smooth granular
livers were seen in a 28-day feeding study in mice. The LOEL was
2,520 ppm (328 mg/kg/day) and the NOEL was 420 ppm (55 mg/kg/day).
EPA believes that there is sufficient evidence for listing norflurazon
on EPCRA section 313 pursuant to EPCRA section 313(d)(2)(B) based
on the available hepatic and thyroid toxicity
data.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
-- A nine-month oral
toxicity study (MRID 00091056) was conducted using the F generation
of rats from a two-year carcinogenicity study (MRID 00082019).
In this study, rats were given technical norflurazon in the diet
at dose levels of 0, 125, 250, or 500 ppm (0, 6.25, 12.5, and
25 mg/kg/day) for 39 weeks... At 500 ppm... Thyroid
weight was decreased in males by approximately
30% vs control,
while
thyroid weight was increased in females between 11-47% vs control.
Gonadal
weight (left gonad) was increased in female rats by 30%.
At 250
ppm, thyroid weight was decreased by 30% in male rats, but was
increased by 11-29% in female rats.
However, there were no reported microscopic alterations in this
organ at any dose level tested. Gonadal
weight in female rats was increased by 17% vs. control.
The systemic NOEL was determined to be 250 ppm (12.5 mg/kg/day)
in both sexes. The systemic LEL was determined to be 500 ppm (25
mg/kg/day) in both sexes, based on the dose-related increase in
liver weight in male and female rats
at 39 weeks, the increase in gonad weight
of females, and the microscopic changes observed in
kidneys of
both sexes. Although
dramatic effects on thyroid weight were
observed at 250 ppm in both sexes, there were no data indicating
any alteration in histology of this organ. Thus, the weight change,
while indicative of an effect of norflurazon, is not supported
as a toxic effect based on available data (guideline: non-guideline
study; classified as core supplementary; MRID 00091056).
-- A chronic toxicity and carcinogenicity study was conducted
in Sprague-Dawley rats. In this study, technical norflurazon was
administered in the diet at dose levels of 0, 125, 375, or 1025
ppm (0, 6.25, 18.75, or 51.25 mg/kg/day) for 104 weeks... The
weight of the thyroid was also increased
at the 1025 ppm dose in male rats
at 104 weeks. An increased incidence of
hydronephrosis was observed in high dose male rats at 52
weeks vs control, while the incidence of
nephritis
was increased
in male rats (terminal sacrifice + dying on test) at the 1025
ppm dose. The incidence of tubular casts was increased in female
rats at the high dose in those rats surviving to study termination.
Other microscopic alterations observed at the high dose included
an increased incidence of parathyroid hyperplasia
(both sexes), hemosiderin pigment deposition in the spleen
(males only) and liver (both sexes), and endometritis
and squamous metaplasia of the uterus (females). The systemic
NOEL was determined to be 375 ppm (18.75 mg/kg/day) for both sexes.
The systemic LEL was determined to be 1025
ppm (51.25 mg/kg/day) in both sexes, based on the increased kidney
weight and accompanying microscopic pathologic changes, as well
as the increase in liver weight in male and female rats
and the increase in thyroid weight in males.
There was no evidence of carcinogenicity for norflurazon (guideline
83-5; MRIDs 00111617 and 00082019). As a result of the July 18,
1990 meeting of the OPP/Health Effects Division Carcinogenicity
Peer Review Committee, norflurazon was classified as a non quantifiable
Group C - possible human carcinogen - based
upon statistically significant pair-wise comparisons of the incidence
of liver adenomas and combined liver adenomas/ carcinomas as well
as statistically positive trends for these lesions in male CD-1
mice receiving 218.8 mg/kg/day norflurazon technical in
the diet for up to 104 weeks (MRID 00111649).
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/PESTICIDES/Norflurazon.RED.EPA.pdf
Endocrine:
Uterus (click
on for all fluorinated pesticides)
-- A two-year carcinogenicity
study was conducted in male and female CD-1 HaM/ICR Swiss mice
in which 125 mice/sex/dose were administered technical norflurazon
in the diet at dose levels of 0, 85, 340, or 1360 ppm (0, 12.8,
58.7, or 218.8 mg/kg/day) for 100-104 weeks...
Increased incidences of enlarged spleen, nephritis, swollen/enlarged
liver, and nodular enlargement of the liver were observed in high
dose male mice, while increased incidences of pyelonephritis,
enlarged liver, and
cystic ovaries were observed in high dose female mice.
Carcinogenic potential was evidenced by an increased incidence
of hepatic adenoma and combined adenoma/carcinoma
in high dose male mice. The systemic NOEL was determined
to be 12.8 mg/kg/day (85 ppm) for male mice, and 58.7 mg/kg/day
(340 ppm) for female mice. The systemic LEL was determined to
be 58.7 mg/kg/day (340 ppm) for male mice, based on the increased
incidence of enlarged spleen, increased
absolute and relative liver weight, and increased incidence of
nephritis. The systemic LEL was determined to be 218.8
mg/kg/day (1360 ppm) for female mice, based on the increased incidence
of enlarged liver and cystic
ovaries, the
increased absolute and relative liver weight,
and the increased incidence of pyelonephritis (guideline §83-2;
MRID 00111649).
-- A chronic toxicity and carcinogenicity study was conducted
in Sprague-Dawley rats. In this study, technical norflurazon was
administered in the diet at dose levels of 0, 125, 375, or 1025
ppm (0, 6.25, 18.75, or 51.25 mg/kg/day) for 104 weeks... Other
microscopic alterations observed at the high dose included an
increased incidence of parathyroid hyperplasia
(both sexes), hemosiderin pigment deposition in the spleen
(males only) and liver (both sexes), and endometritis
and squamous metaplasia of the uterus (females). The
systemic NOEL was determined to be 375 ppm (18.75 mg/kg/day) for
both sexes. The systemic LEL was determined to be 1025 ppm (51.25
mg/kg/day) in both sexes, based on the increased kidney
weight and accompanying microscopic pathologic changes, as well
as the increase in liver weight in male and female rats
and the increase in thyroid weight in males.
There was no evidence of carcinogenicity for norflurazon (guideline
83-5; MRIDs 00111617 and 00082019). As a result of the
July 18, 1990 meeting of the OPP/Health Effects Division Carcinogenicity
Peer Review Committee, norflurazon was classified as a non quantifiable
Group C - possible human carcinogen - based
upon statistically significant pair-wise comparisons of the incidence
of liver adenomas and combined liver adenomas/ carcinomas as well
as statistically positive trends for these lesions in male CD-1
mice receiving 218.8 mg/kg/day norflurazon technical in
the diet for up to 104 weeks (MRID 00111649).
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/PESTICIDES/Norflurazon.RED.EPA.pdf
Kidney
(click
on for all fluorinated pesticides)
-- A two-year carcinogenicity
study was conducted in male and female CD-1 HaM/ICR Swiss mice
in which 125 mice/sex/dose were administered technical norflurazon
in the diet at dose levels of 0, 85, 340, or 1360 ppm (0, 12.8,
58.7, or 218.8 mg/kg/day) for 100-104 weeks... The systemic LEL
was determined to be 58.7 mg/kg/day (340 ppm) for male mice, based
on the increased incidence of enlarged spleen,
increased absolute and relative liver weight,
and increased incidence of nephritis...
-- A chronic toxicity and carcinogenicity study was conducted
in Sprague-Dawley rats. In this study, technical norflurazon was
administered in the diet at dose levels of 0, 125, 375, or 1025
ppm (0, 6.25, 18.75, or 51.25 mg/kg/day) for 104 weeks...
The systemic LEL was determined to be 1025 ppm (51.25 mg/kg/day)
in both sexes, based on the increased kidney
weight and accompanying microscopic pathologic changes,
as well as the increase in liver weight
in male and female rats and the increase in thyroid weight
in males...
-- A 2-generation reproductive toxicity study was conducted in
male and female Wistar rats. In this study, norflurazon technical
was administered in the diet at dose levels of 0, 150, 750, or
1500 ppm over 2 generations (0, 10.2, 50.8, or 102.5 mg/kg/day
for F males; 0, 12.1, 0 62.0, or 129.7 mg/kg/day for F females;
0, 13.2, 67.8, or 138.6 0 mg/kg/day for F males; 17.1, 81.7, and
173.0 mg/kg/day for F1 1 females). The NOEL for systemic toxicity
was determined to be 150 ppm (10.2 mg/kg/day for males and 12.1
mg/kg/day for females), and the systemic toxicity LEL was determined
to be 750 ppm (50.8 mg/kg/day for males and 62.0 mg/kg/day for
females), based on significant increases
in liver and kidney weights observed in both generations
of parental rats and the increased incidence of hepatocellular
hypertrophy in both generations of parental
rats...
-- A nine-month oral toxicity study (MRID 00091056) was conducted
using the F generation of rats from a two-year carcinogenicity
study (MRID 00082019). In this study, rats were given technical
norflurazon in the diet at dose levels of 0, 125, 250, or 500
ppm (0, 6.25, 12.5, and 25 mg/kg/day) for 39 weeks... Kidney weight
was not significantly affected, but the incidence of hyaline
pigment deposition (many tubules) in male rats and hyaline pigment
deposition (few tubules) in female rats was increased,
as was the incidence of medullary congestion
in high dose female rats. The incidence of tubular
degeneration was increased in both sexes at the 500 ppm
dose level. ... The systemic LEL was determined to be 500 ppm
(25 mg/kg/day) in both sexes, based on the dose-related increase
in liver weight in male and female rats at 39 weeks, the increase
in gonad weight of females, and the microscopic
changes observed in kidneys of both sexes. Although dramatic
effects on thyroid weight were observed at 250 ppm in both sexes,
there were no data indicating any alteration in histology of this
organ. Thus, the weight change, while indicative of an effect
of norflurazon, is not supported as a toxic effect based on available
data (guideline: non-guideline study; classified as core supplementary;
MRID 00091056).
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/PESTICIDES/Norflurazon.RED.EPA.pdf
Liver
(click
on for all fluorinated pesticides)
Group
C -- Possible Human Carcinogen. Statistically
significant increase in comparison to controls in liver
adenomas & combined liver adenomas & carcinomas, as well
as the statistically significant positive trend for these hepatocellular
adenomas & combined adenomas & carcinomas; CD-1 mice (M).
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
-- A two-year carcinogenicity
study was conducted in male and female CD-1 HaM/ICR Swiss mice
in which 125 mice/sex/dose were administered technical norflurazon
in the diet at dose levels of 0, 85, 340, or 1360 ppm (0, 12.8,
58.7, or 218.8 mg/kg/day) for 100-104 weeks... Liver
weight was increased by 9% and 15% in male and female mice at
the 340 ppm dose level, and by 27% and 21% at the 1360 ppm dose
level, respectively. The liver to
body weight ratio was increased by 19% and 4% in male and female
mice at the 340 ppm dose level, and by 43% and 19% at the 1360
ppm dose level, respectively. Increased incidences of
enlarged spleen, nephritis, swollen/enlarged
liver, and nodular enlargement of the liver were observed in high
dose male mice, while increased incidences of
pyelonephritis, enlarged
liver, and cystic ovaries were observed
in high dose female mice. Carcinogenic
potential was evidenced by an increased incidence of hepatic
adenoma and combined adenoma/carcinoma in high dose male mice.
The systemic NOEL was determined to be 12.8 mg/kg/day (85 ppm)
for male mice, and 58.7 mg/kg/day (340 ppm) for female mice. The
systemic LEL was determined to be 58.7 mg/kg/day (340 ppm) for
male mice, based on the increased incidence of
enlarged spleen,
increased absolute and relative liver weight,
and increased incidence of nephritis.
The systemic
LEL was determined to be 218.8 mg/kg/day (1360 ppm) for female
mice, based on the increased incidence of
enlarged liver and cystic ovaries,
the increased
absolute and relative liver weight,
and the increased incidence of pyelonephritis
(guideline §83-2; MRID 00111649).
-- As
a result of the July 18, 1990 meeting of the OPP/Health
Effects Division Carcinogenicity Peer Review Committee, norflurazon
was classified as a non quantifiable Group C - possible
human carcinogen - based upon statistically significant
pair-wise comparisons of the incidence of liver
adenomas and combined liver adenomas/ carcinomas as well as statistically
positive trends for these lesions in male CD-1 mice receiving
218.8 mg/kg/day norflurazon technical in the diet for up to 104
weeks (MRID 00111649).
-- A 2-generation reproductive toxicity study was conducted in
male and female Wistar rats. In this study, norflurazon technical
was administered in the diet at dose levels of 0, 150, 750, or
1500 ppm over 2 generations (0, 10.2, 50.8, or 102.5 mg/kg/day
for F males; 0, 12.1, 0 62.0, or 129.7 mg/kg/day for F females;
0, 13.2, 67.8, or 138.6 0 mg/kg/day for F males; 17.1, 81.7, and
173.0 mg/kg/day for F1 1 females). The NOEL for systemic toxicity
was determined to be 150 ppm (10.2 mg/kg/day for males and 12.1
mg/kg/day for females), and the systemic toxicity LEL was determined
to be 750 ppm (50.8 mg/kg/day for males and 62.0 mg/kg/day for
females), based on significant increases
in liver and kidney weights observed in both generations
of parental rats and the increased incidence of hepatocellular
hypertrophy in both generations of parental rats...
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/PESTICIDES/Norflurazon.RED.EPA.pdf
Congestion of the
liver, hepatocyte swelling and increased liver weights,
and increase in colloid vacuole in the thyroid
were observed in dogs fed 450 ppm (10.25 mg/kg/day) norflurazon
for 6 months. The NOEL was 150 ppm (3.75 mg/kg/day). An oral RfD
of 0.04 mg/ kg/day has been determined. Increased relative liver
weight and hypertrophy of the thyroid with depletion of
colloid were seen in rats fed 2,500 ppm (125 mg/kg/day) norflurazon
for 90 days. The NOEL was 500 ppm (25 mg/kg/day). Hepatic
hyperplasia and hypertrophy and increased relative liver weight
were noted in a 28-day feeding study in rats. The LOEL was 1,000
ppm (50 mg/kg/day) and the NOEL was 500 ppm (25 mg/kg/ day). Increased
relative liver weight and diffuse and smooth
granular livers were seen in a 28-day feeding study in
mice. The LOEL was 2,520 ppm (328 mg/kg/day) and the NOEL was
420 ppm (55 mg/kg/day). EPA believes that there is sufficient
evidence for listing norflurazon on EPCRA section 313 pursuant
to EPCRA section 313(d)(2)(B) based on the available
hepatic and thyroid toxicity data.
Ref: USEPA/OPP. Support Document for the
Addition of Chemicals from Federal Insecticide, Fungicide, Rodenticide
Act (FIFRA) Active Ingredients to EPCRA Section 313. U. S. Environmental
Protection Agency, Washington, DC (1993). As cited by US EPA in:
Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
The chronic NOAEL of
1.5 mg/kg/day was established based on increased absolute and
relative liver weight and increased
cholesterol in both sexes in a 6-month feeding study in dogs at
the LOAEL of 4.77 mg/kg/day... The acute and chronic dietary exposure
analyses are based on the Dietary Exposure Evaluation Model (DEEM
TM .) The acute dietary exposure estimates used the entire distribution
of single day food consumption. The chronic dietary exposure estimates
the three day average consumption for each population subgroup.
The DEEM TM analysis was conducted using consumption data from
the USDA 1989-92 Continuing Surveys for Food Intake by Individuals
(CSFII).
Ref: US EPA May 31, 2002. Tolerance Reassessment
Progress and Risk Management Decision.
http://www.fluoridealert.org/pesticides/Norflurazon.TRED.May31.2002.pdf
Spleen
(click
on for all fluorinated pesticides)
- A two-year carcinogenicity
study was conducted in male and female CD-1 HaM/ICR Swiss mice
in which 125 mice/sex/dose were administered technical norflurazon
in the diet at dose levels of 0, 85, 340, or 1360 ppm (0, 12.8,
58.7, or 218.8 mg/kg/day) for 100-104 weeks. No significant effects
were observed on body weight, body weight gain, clinical toxicity,
or food consumption at any dose level tested.
Liver weight was increased by 9% and 15% in male and female mice
at the 340 ppm dose level, and by 27% and 21% at the 1360 ppm
dose level, respectively. The liver to body weight ratio was increased
by 19% and 4% in male and female mice at the 340 ppm dose level,
and by 43% and 19% at the 1360 ppm dose level, respectively. Increased
incidences of enlarged spleen, nephritis,
swollen/enlarged liver, and nodular enlargement of the liver were
observed in high dose male mice,
while increased incidences of pyelonephritis, enlarged liver,
and cystic ovaries were observed in high dose female mice. Carcinogenic
potential was evidenced by an increased incidence of hepatic adenoma
and combined adenoma/carcinoma in high dose male mice. The
systemic NOEL was determined to be 12.8 mg/kg/day (85 ppm) for
male mice, and 58.7 mg/kg/day (340 ppm) for female mice. The systemic
LEL was determined to be 58.7 mg/kg/day (340 ppm) for male mice,
based on the increased incidence of enlarged
spleen, increased absolute and relative
liver weight, and increased incidence of nephritis.
The systemic LEL was determined to be 218.8 mg/kg/day (1360 ppm)
for female mice, based on the increased incidence of enlarged
liver and cystic ovaries, the increased absolute and relative
liver weight, and the increased incidence of pyelonephritis (guideline
§83-2; MRID 00111649).
-- A chronic toxicity and carcinogenicity study was conducted
in Sprague-Dawley rats. In this study, technical norflurazon was
administered in the diet at dose levels of 0, 125, 375, or 1025
ppm (0, 6.25, 18.75, or 51.25 mg/kg/day) for 104 weeks.,, Other
microscopic alterations observed at the high dose included an
increased incidence of parathyroid hyperplasia
(both sexes), hemosiderin pigment deposition in the spleen
(males only) and liver (both sexes), and endometritis
and squamous metaplasia of the uterus (females). The
systemic NOEL was determined to be 375 ppm (18.75 mg/kg/day) for
both sexes...
Ref: US EPA REREGISTRATION ELIGIBILITY DECISION
NORFLURAZON. LIST A. CASE 0229.
http://www.fluoridealert.org/PESTICIDES/Norflurazon.RED.EPA.pdf
| Environmental
(click on for
all fluorinated pesticides)
Norflurazon
- which was on a low-priority sampling list in California
because it was not anticipated to pollute groundwater -
was found in groundwater by Florida which is more vigilant
about water quality because the state is experiencing a
water pollution crisis. California found norflurazon - the
third most popular herbicide used on the state's roadsides
- in 9.5% of the wells in 1997, the first time samples
were taken.
Ref: Pathways
of Exposure. Californians for
Alternatives to Toxics.
Drinking
water exposure to pesticides can occur through surface and/or
ground water contamination. The Agency considers acute (one
day) and chronic (lifetime) drinking water risks and uses
either modeling or actual monitoring data, if available,
to estimate these risks. Modeling is carried out in tiers
of further refinement, and is designed to provide a high
end estimate of exposure. Norflurazon is resistant to abiotic
hydrolysis and has a relatively low volatilization potential.
Norflurazon is relatively resistant to microbial degradation
with aerobic and anaerobic half lives of 130 day (aerobic
soil metabolism study), 6-8 months (aerobic aquatic metabolism
study), and an 8 month (anaerobic aquatic metabolism study).
The primary microbial degradate is desmethyl norflurazon.
The relatively low soil/water partitioning of norflurazon
indicates that norflurazon can leach to ground water and
runoff will generally be via dissolution rather than absorption
to eroding soil. The drinking water residues of concern
are norflurazon and desmethyl norflurazon. Norflurazon has
been detected in ground water in Florida and North Carolina.
According to the 1995 EFGWB Science Chapter, parent norflurazon
was detected in groundwater Polk County, Florida at concentrations
up to 64 ppb for both acute and chronic. In a monitoring
study in North Carolina, norflurazon was detected in groundwater
in concentrations ranging from 1.5 -5.3 ppb. There have
been reports of norflurazon detected in groundwater in non-target
studies in several states. Although these non-target monitoring
studies do not link pesticide application to the monitoring
data, it is difficult to determine the accuracy of the results.
Two new prospective groundwater (PGW) studies were conducted
in Georgia and Florida to estimate environmental concentration
(EEC) in groundwater for parent norflurazon and desmethyl
norflurazon. PGW studies are targeted monitoring studies
that link to known use areas and application rates in evaluating
the potential for leaching to groundwater. The groundwater
EEC for norflurazon is based on concentrations from the
PGW studies of 29.9 ppb for norflurazon and 23.8 ppb for
desmethyl norflurazon. The groundwater EECs are 53.7 ppb
for acute exposure and 15 ppb for chronic exposure. These
groundwater EECs are less than the Agency’s Drinking Water
Levels of Concern (DWLOC) for both acute and chronic dietary
risk. There is limited surface water monitoring information
available from USGS studies, but the monitoring was not
from targeted use areas and parent compound only was measured
in some of the studies. Therefore, the available monitoring
data is not sufficient for a drinking water assessment.
EECs were calculated using Tier II modeling tools (PRZM-EXAMS/IR)
for norflurazon and the Tier I modeling tool (FIRST) for
desmethyl norflurazon. Total estimated residues were summed
from the two models giving a peak value of 633 ppb for acute
exposures and a one in ten year annual average concentration
of 185 ppb for chronic exposures. The surface water EEC
is less than the DWLOC for acute dietary risk. However,
the surface water EEC for chronic dietary risk slightly
exceeds the DWLOC for infants and children 1-12...
While the surface water EEC apparently slightly exceeds
the chronic DWLOC for infant and children sub-populations,
this apparent exceedance is not considered to be a concern
based on conservative assumptions used in modeling the chronic
EECs.
Ref: US EPA May 31, 2002.
Tolerance Reassessment Progress and Risk Management Decision.
http://www.fluoridealert.org/pesticides/Norflurazon.TRED.May31.2002.pdf
|
|

