|
Return
to Index Page
Because EPA stated the following,
we will combine the adverse effects for Mefluidide, and its diethanolamine
and potasium salts.
Based on the structural similarities of mefluidide
and its diethanolamine (DEA) and potassium salts, where they
all share the same anion- anilide, and the physical and chemical
properties of the DEA and potassium salts, where they dissociate
100% back to free mefluidide in aqueous environments, the
risk assessment team concluded that mefluidide DEA and potassium
salts are biologically equivalent to mefluidide and thus they
share the same toxicity as the free mefluidide. Therefore,
it is reasonable to bridge mefluidide toxicity data to mefluidide
salts and vice versa.
Reference: USEPA. Mefluidide;
Diethanolamine Mefluidide, and Potassium Mefluidide- Phase 2,
(30-Day Error only Correction), HED Chapter of the Re-registration
Eligibility Decision Document (RED). May
30, 2007.
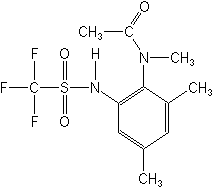
MEFLUIDIDE
ACTIVITY:
Herbicide, Plant growth regulator (anilide)
CAS Name: N-[2,4-dimethyl-5-[[(trifluoromethyl)sulfonyl]amino]phenyl]acetamide
Structure:
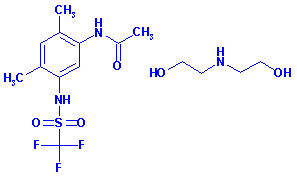
DIETHANOLAMINE
SALT OF MEFLUIDIDE
CAS No.
53780-36-2
Systematic Name: Acetamide, N-(2,4-dimethyl-5-(((trifluoromethyl)sulfonyl)amino)phenyl)-,
compd. with 2,2'-iminobis(ethanol) (1:1)
Activity: Herbicide (Anilide)
Structure:
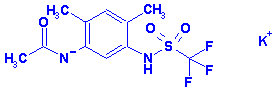
MEFLUIDIDE,
POTASSIUM SALT
CAS NO.: 83601-83-6
ACTIVITY: Herbicide (anilide)
Systematic Name: Acetamide, N-(2,4-dimethyl-5-(((trifluoromethyl)sulfonyl)amino)phenyl)-,
monopotassium salt
Structure:
Adverse Effects:
Body
Weight Decrease
Developmental
Eye
Kidney
Liver
Sciatic nerve
Spleen
Environmental:
• Moderately persistent and mobile in terrestrial environments.
• Above the USEPA's
level of concern for direct
acute (listed and nonlisted) and chronic toxic exposure to mammals,
birds and acute (listed and nonlisted) exposure to terrestrial and
semi aquatic plants.
The highest
use areas for mefluidide include South
Carolina, North Carolina, Virginia, West Virginia, California,
Nevada, Arizona, and New Mexico. The maximum application
rate for mefluidide applied as ground sprays is 1.0 lb ae/A
for mefluidide-K and mefluidide-DEA. The maximum application
rate for mefluidide, as a granular formulation, is 0.5 lb
ae/A. Mefluidide, mefluidide-K, mefluidide-DEA can be applied
3 times per season.
Reference: USEPA.
Re-registration Eligibility Document Environmental Fate
and Effects Science Chapter. June 9, 2007.
- http://www.fluoridealert.org/pesticides/docket/EPA-HQ-OPP-2007-0431-0017.pdf
Cortical
necrosis in kidney? brain? bone?
FAN will ask EPA to clarify:
Feeding
[dog]. LOAEL = 15 mg/kg/d, based on decreased body weight
(15%) and body weight gain (50%) in the males. Chronic
cortical nephrosis was observed at 150 mg/kg/day
dose. NOAEL = 1.5 mg/kg/d
Ref:
USEPA. Mefluidide
- Toxicology section for the Reregistration Eligibility
Decision Document (RED) (January 31, 2007) - http://www.fluoridealert.org/pesticides/docket/EPA-HQ-OPP-2007-0431-0009.pdf
|
Body
Weight
(click
on for all fluorinated pesticides)
• Rat: Non-guideline range finding developmental toxicity,
gavage with diethanolamine salt of mefluidide. Developmental LOAEL:
230 mg a.i. /kg/day based on significantly decreased fetal body
weight. Developmental NOAEL: 173 mg a.i. /kg/day, The dosage levels
of 0, 50, 200 and 400 mg of the 28.78% formulation/kg/day weredefinitive
developmental study.
• Dog: A NOAEL of 1.5 mg/kg/day was selected based on chronic
toxicity (decreased body weight (15%)
and body weight gain (50%)
in the males) occurring at a LOAEL
of 15.0 mg/kg/day. This was the most sensitive
endpoint. An UF of 100X (10-fold for interspecies extrapolation,
10-fold for intraspecies variability) was applied to the NOAEL
of 1.5 mg/kg/day to derive the cRfD to give and RfD of 0.015 mg/kg/day.
• In rats and rabbits, critical effects of acute oral toxicity
occurring at doses of 100 mg/kg/day and above were tremors, hunched
posture, salivation, reduced body weight
and body weight gain.
• Mefluidide and its diethanolamine
salt subchronic and chronic toxicity are manifested
by decreased body weight and body weight gain in several
species tested (rats, rabbits and dogs). Dogs are most sensitive
to these effects, which occur at doses as low as 15 mg/kg/day
in diets fed for one year.
• Rats: The maternal toxicity included tremors, decreased
body weight, weight gain and mortality.
• In the 2-generation reproduction toxicity study, the offspring
toxicity was characterized by decreased
body weights in both sexes and both litters in all generations.
The reproductive LOAEL was not observed (NOAEL = 346/604
mg/kg bw/day).
• Evidence of maternal toxicity included transient clinical
signs (tremors, dark material around the nose, few feces, urine
stain and reddish vaginal discharge), decreased
body weight gain (11-61%)
•
At 6000 ppm, body weights were decreased by 1-8%
in males and 1-12% in females
throughout the study in the P generation, attaining
significance (p<0.05) at Week 18 in the males and Weeks
8, 18, 19, and 27 in the females. In the F1 generation at this
dose, body weights were decreased throughout
the study in the males (decr. 13-21%) and females
(decr. 10-21%), attaining significance (p<0.01) at Weeks 27,
37, and 56 in both sexes. Similarly in the F2 generation,
body weights were decreased throughout the
study in the 6000 ppm males (decr. 14-21%) and females (decr.
11-23%), attaining significance (p<0.01)
at Weeks 57, 66, and 85 in both sexes.
• Numerous absolute and relative
(to bw) organ weights in the 6000 ppm parents were significantly
(p<0.05) different from the controls, however,
none of these differences were corroborated by any macroscopic
or microscopic findings indicating these decreases were most likely
not related to treatment. Thus, it is likely that they were attributable
to decreased body weights at this dose.
Ref: USEPA.
Mefluidide - Toxicology section for the Reregistration Eligibility
Decision Document (RED) (January 31, 2007)
http://www.fluoridealert.org/pesticides/docket/EPA-HQ-OPP-2007-0431-0009.pdf
Developmental
(click
on for all fluorinated pesticides)
Developmental effects of Mefluidide in rats included increased
number of early resorptions and mean postimplantation
loss. These effects were observed at the same dose that
caused maternal toxicity indicating there was no increased susceptibility
to fetuses. The maternal toxicity included tremors, decreased
body weight, weight gain and mortality. In rabbit, the LOAEL/NOAEL
for developmental toxicity were above the highest dose tested
(60 mg/kg/day). In the 2-generation reproduction toxicity study,
the offspring toxicity was characterized by decreased body weights
in both sexes and both litters in all generations. The reproductive
LOAEL was not observed (NOAEL = 346/604 mg/kg bw/day).
Ref:
USEPA. Mefluidide - Toxicology
section for the Reregistration Eligibility Decision Document (RED)
(January 31, 2007)
http://www.fluoridealert.org/pesticides/docket/EPA-HQ-OPP-2007-0431-0009.pdf
Eye
(click
on for all fluorinated pesticides)
The studies reviewed
below were conducted with either the free acid or the diethanolamine
salt of mefluidide. The diethanolamine salt is
the registered active ingredient in California. Possible toxicological
differences between the free acid and diethanolamine salt were
not considered in the following reviews. The free acid and diethanolamine
salt have been grouped by the US EPA (Morris, 10/5/93)...
-- 386-004 987263, "Two Year Feeding study in Rats", (International
Research and Development Corporation, Mattawan, MI, study no.
102-208, report no. 225, 6/14/79). Mefluidide (MBR 12325) technical,
purity 93%, fed in the diet to 50/sex/group at 0, 600, 1800 or
6000 ppm over two (2) years starting at in utero from a reproductive
study. ADVERSE EFFECT: retinal degeneration.
Eye effect NOEL = 600 ppm.
Ref: Summary of Toxicology Data. 11-21-94
Revision. California EPA, Department of Pesticide Regulation,
Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Mefluidide.CA.EPA.1986.pdf
Kidney
(click
on for all fluorinated pesticides)
The studies reviewed
below were conducted with either the free acid or the diethanolamine
salt of mefluidide. The diethanolamine
salt is the registered active ingredient in California.
Possible toxicological differences between the free acid and diethanolamine
salt were not considered in the following reviews. The free acid
and diethanolamine salt have been grouped by the US EPA (Morris,
10/5/93)...
-- ** 386-026 037397, "Twelve Month Diet Feeding Study of MBR-12325
in Dogs", (Riker Laboratories, experiment no. 0280CD0021, 11/1/82).
Technical MBR-12325 (Mefluidide), purity 93% and 91% at pre-study
and post-study, respectively, fed at 0, 60, 600 or 6000 ppm in
the diet to Beagle dogs, 6/sex/group for one year. Positive
for adverse effects on kidney at high dose. NOEL kidney
= 600 ppm; body weight = 60 ppm. Nephrosis
or degeneration of proximal convoluted renal tubular epithelium
in 4/12 at high dose. Record 059988 contains the diet analyses
for content, stability and homogeneity. ACCEPTABLE. (Gee, 3/17/86
and 7/14/88). EPA one-liner: Minimum. NOEL = 60 ppm (weight loss);
LEL = 600 ppm (cortical nephrosis). EPA one-liner: Minimum. NOEL
= 60 ppm (weight loss); LEL = 600 ppm (cortical nephrosis).
Ref: Mefluidide, diethanolamine salt: Summary
of Toxicology Data. 11-21-94
Revision. California EPA,
Department of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Mefluidide.CA.EPA.1986.pdf
Liver
(click
on for all fluorinated pesticides)
"Lifetime Carcinogenicity Study in Mice", (International
Research and Development Corporation, report no. 102-026, 5/14/1979).
Mefluidide, purity 93%, fed at 0, 600, 1800 or 6000 ppm in diet
to 60/sex/group for 18-19 months. Positive
adverse chronic effect of liver toxicity. No evidence of
oncogenicity. NOEL for hepatotoxicity is 600 ppm. UNACCEPTABLE.
(J. Christopher, 7/1/85). Evaluated as possibly upgradeable with
submission of pathology data adjusted according to time of death,
complete histopathology for the mid dose group and organ weights.
Rebuttal (#059987 in 034) discusses the requirements for an oncogenicity
study as opposed to a combined and contains diet analyses. See
Summary statement below. (Updated, Kishiyama and Gee, 7/14/88).
...Significant adverse effects consisted of increased mortality
in the 6000 ppm (high) dose group and liver
nodular hyperplasia in the 6000 and 1800 (intermediate) dose groups.
The liver alterations were called "reparative or regenerative
responses to toxic liver injury" and no oncogenic
effect was claimed. Two consultant pathologist re-read the slides
and generally agreed with the diagnoses of the IRDC pathologist.
While no oncogenic effects were found, the
reduced survival in the high dose group confounds interpretation.
The pathology data need to be adjusted according to time of death
rather than be lumped into singular incidence values irrespective
of time of death or sacrifice. Appropriate statistics could then
be performed. In any event, the report is still incomplete and
unacceptable due to study conduct deficiencies originally outlined
in Dr. Christopher’s review. F. Martz, 4/16/86.
Ref:
Summary of Toxicology Data. 11-21-94 Revision. California EPA,
Department of Pesticide Regulation, Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Mefluidide.CA.EPA.1986.pdf
21-Day Dermal toxicity - rabbit (4 rabbits/sex/dose). Acceptable/Non-guideline
(NOAEL was not observed). Systemic LOAEL = 240 mg/kg/day, based
on clinical chemistry (increased alkaline phosphatase and alanine
aminotransferase) and organ weights (decreased spleen weight in
females and increased liver weights in males).
Edema and swelling with myelin loss in sciatic nerve was seen
in 720 and 2400 mg/kg/day dose group. Dehydration observed at
2400 mg/kg/day dose. Dermal and systemic NOAELs were not established.
Ref:
USEPA. Mefluidide - Toxicology
section for the Reregistration Eligibility Decision Document (RED)
(January 31, 2007)
http://www.fluoridealert.org/pesticides/docket/EPA-HQ-OPP-2007-0431-0009.pdf
Sciatic
nerve (click
on for all fluorinated pesticides)
21-Day Dermal toxicity - rabbit (4 rabbits/sex/dose). Acceptable/Non-guideline
(NOAEL was not observed). Systemic LOAEL = 240 mg/kg/day, based
on clinical chemistry (increased alkaline phosphatase and alanine
aminotransferase) and organ weights (decreased spleen weight in
females and increased liver weights in males).
Edema and swelling with myelin loss in sciatic
nerve was seen in 720 and 2400 mg/kg/day dose group. Dehydration
observed at 2400 mg/kg/day dose. Dermal and systemic NOAELs were
not established.
Ref:
USEPA. Mefluidide - Toxicology
section for the Reregistration Eligibility Decision Document (RED)
(January 31, 2007)
http://www.fluoridealert.org/pesticides/docket/EPA-HQ-OPP-2007-0431-0009.pdf
Spleen
(click
on for all fluorinated pesticides)
21-Day Dermal toxicity - rabbit (4 rabbits/sex/dose). Acceptable/Non-guideline
(NOAEL was not observed). Systemic LOAEL = 240 mg/kg/day, based
on clinical chemistry (increased alkaline phosphatase and alanine
aminotransferase) and organ weights (decreased
spleen weight in females and increased
liver weights in males). Edema and
swelling with myelin loss in sciatic nerve was seen in 720 and
2400 mg/kg/day dose group. Dehydration observed at 2400
mg/kg/day dose. Dermal and systemic NOAELs were not established.
Ref:
USEPA. Mefluidide - Toxicology
section for the Reregistration Eligibility Decision Document (RED)
(January 31, 2007)
http://www.fluoridealert.org/pesticides/docket/EPA-HQ-OPP-2007-0431-0009.pdf
| Environmental
(click
on for all fluorinated pesticides)
Based on the review of the environmental
fate data, mefluidide is moderately
persistent and mobile in terrestrial environments.
Possible routes of dissipation for mefluidide are photodegradation
on soil surfaces, microbial mediated degradation, leaching,
and runoff. There are no aerobic aquatic metabolism data to
assess the environmental fate of mefluidide in aquatic environments.
1.2 Potential Risk to Non Target Organisms
This screening-level (Level I) risk assessment focused on
the use of mefluidide-K, mefluidide-DEA, and mefluidide on
ornamental and turf areas. Results suggest
that levels of mefluidide in the environment, when compared
with measured toxicity for the most sensitive organisms of
selected taxa, are likely to result in direct risks to listed
and non-listed species from several different taxa.
Indirect risks are also identified for listed and non-listed
non-target organisms.
This screening level risk assessment shows
that use of mefluidide is below the Agency’s level of
concern for direct acute (listed and non-listed) and chronic
toxic exposure to aquatic freshwater and estuarine marine
organisms and acute aquatic plants. In contrast, the
use of mefluidide is above the Agency’s level of concern
for direct acute (listed and nonlisted) and chronic toxic
exposure to mammals, birds and acute (listed and nonlisted)
exposure to terrestrial and semi aquatic plants.
The results of this risk assessment suggest
that the patterns of mefluidide use are such that they coincide
in time and space to areas frequented by avian and mammalian
wildlife. These areas have been demonstrated as use by wildlife
as sources of food and cover. The potentially problematic
wildlife food items suggested by this risk assessment are
likely to be present in and around the treated areas. In addition,
there is potential for indirect effects to all taxonomic groups
due to changes in habitat caused by vegetation changes. Some
uses of mefluidide may not pose a threat for avian and mammalian
wildlife, such as industrial sites that are
not frequented by wildlife
• Mammalian Acute Listed LOCs were exceeded
for 15 g and 35 g mammals exposed to application rates for
mefluidide-DEA and mefluidide-K (1.0 lb ae/A at 3 applications)
consuming short grass, broadleaf plants, or small insects
and 1000 g mammals that consume short grass.
• Mammalian Acute Listed LOCs were exceeded for the
LD50s/sq-ft for 15g and 35 g mammals based on one granular
application of mefluidide at 0.5 lbs ae/acre.
• Mammalian Acute Restricted Use LOCs were exceeded
for 15 g and 35 g mammals that consume short grass exposed
to application rates for mefluidide-DEA and mefluidide-K (
1.0 lb ae/A at 3 applications).
• Mammalian Acute Restricted Use LOCs were exceeded
for the LD50s/sq-ft for small and medium-sized mammals based
on one granular application of mefluidide at 0.5lbs ae/acre.
• Mammalian Chronic LOCs (dose-based) were exceeded
for 15 g mammals that consume short grass exposed to application
rates for mefluidide-DEA and mefluidide-K (1.0 lb ae/A at
3 applications)
• Avian Acute Listed LOCs were exceeded for 20 g birds
that consume short grass, tall grass and broadleaf plants
and small insects and 100 g birds that consume short grass
for the 1.0 lb ae/A modeled scenario. Non-definitive toxicity
endpoints do not allow for calculations of definitive RQs,
however the ratio of non- definitive endpoints (EECs) in this
case results in acute RQs ranging from <0.0 to <0.25.
• Avian Acute Listed LOCs were exceeded for the LD50s/sq-ft
for 20 g birds based on one granular application of mefluidide
at 0.5 lbs ae/acre.
• Avian Acute Restricted Use LOCs were exceeded for
20 g birds that consume short grass for the 1.0 lba ae/A application
rate modeled scenario. Non-definitive toxicity endpoints do
not allow for calculations of definitive RQs, however the
ratio of non- definitive endpoints (EECs) in this case results
in acute RQs of < 0.25.
• Avian Acute Restricted Use LOCs were exceeded for
the LD50s/sq-ft for 20 g birds based on one granular application
of mefluidide at 0.5 lbs ae/acre.
• Avian Chronic LOCs (dietary-based) exceedances occurred
for birds for the 1.0 lb ae/A modeled scenario. Non-definitive
toxicity endpoints do not allow for calculations of definitive
RQs, however the ratio of non- definitive endpoints (EECs)
in this case results in acute RQs ranging from 2.9 to 6.32.
• Terrestrial and Semi-aquatic Plants (Listed Species
and Non-Listed Species) LOCs were exceeded for monocots and
dicots with the 1.0 lb ae/A spray applications of mefluidide-K
and mefluidide-DEA. LOCs were exceeded for dicots and monocots
(granular applications) with 0.5 lb ae/acre of mefluidide.
Dicots demonstrated more sensitivity than monocots in all
application scenarios.
• The available dietary toxicity studies
on avian species failed to established definitive acute LD50
values (i.e., the lethality values exceed the highest dose
tested). Therefore, use of this value adds uncertainty and
may overestimate risk to avian species. Submission
of a acute bird study with definitive LD50 values would quantify
risks associated with exposure of mefluidide to birds.
• Exposure estimates for this screening
level risk assessment focused on the mefluidide, mefluidide-K
and mefluidide-DEA. Information or data is not available to
evaluate degradates as a potentially significant contributor
to aquatic risk and which may affect the outcome of risk conclusions
are not considered in this risk characterization. Therefore,
this assessment may require further analysis to evaluate degradates
as a potential contributor to aquatic risk.
• In all cases, EFED concluded that
resulting estimated risk quotients, had they been based on
definitive effects measurement endpoints, would not trigger
concerns for acute or chronic risks to freshwater fish, chronic
estuarine marine fish, chronic estuarine marine invertebrates,
chronic freshwater invertebrates, vascular plants (EC05 or
NOAEC) and non-vascular plants (EC05 or NOAEC). In contrast,
EFED concluded that resulting estimated risk quotients for
terrestrial organisms would trigger concerns for chronic risks
to birds and (listed and nonlisted) terrestrial and semi aquatic
plants.
• . Information or data was not available
to evaluate degradates as a potentially significant contributor
to aquatic risk and is not considered in this risk characterization.
• A major uncertainty in the assessment
is the persistence of mefluidide acid in aerobic aquatic environments.
• Based on the risk hypothesis terrestrial
organisms (birds, mammals, reptiles, terrestrial-phase amphibians
and plants) and aquatic organisms (invertebrates, fish, amphibians
and plants) in surface waters (freshwater or saltwater) are
subject to adverse effects when exposed to mefluidide residues
as a result of labeled use of the pesticide. Routes of exposure
evaluated in this risk assessment focused on runoff and spray
drift from ground spray with mefluidide applied at application
rates of 1.0 lb ae/A (mefluidide-K and mefluidide-DEA) and
runoff from granular applications with 0.5 lb ae/A mefluidide.
•• Ecotoxicity data for chronic
risks to freshwater fish and freshwater invertebrates exposed
to mefluidide were not available.
•• Ecotoxicity data for chronic risks to estuarine
marine fish and estuarine marine invertebrates exposed to
mefluidide were not available.
•• Ecotoxicity data for chronic risks to birds
exposed to mefluidide were not available.
•• The magnitude of toxicity to terrestrial plants
is uncertain because only one terrestrial vegetative vigor
plant study was available and conducted on fresh weight and
not dry weight as required by EPA guidelines. Also, no other
terrestrial plant toxicity studies (seedling emergence and
vegetative vigor) were available to estimate an EC25 values.
Ecotoxicity data for terrestrial plants (seedling emergence)
exposed to mefluidide were not available.
•• NOAEC or EC05 values were not available to
calculate (listed) aquatic vascular plants exposed to mefluidide.
•• The available dietary toxicity studies on avian
species failed to established definitive acute LD50 values
(i.e., the lethality values exceed the highest dose tested).
•• Exposure estimates for this screening level
risk assessment focused on the mefluidide, mefluidide-K and
mefluidide-DEA. Information or data is not available to evaluate
degradates as a potentially significant contributor to aquatic
risk and which may affect the outcome of risk conclusions
are not considered in this risk characterization. Therefore,
this assessment may require further analysis to evaluate degradates
as a potential contributor to aquatic risk.
•• This risk assessment does not consider incidental
soil ingestion.
•• The screening assessment does not consider
dermal exposure.
Reference: USEPA.
Re-registration Eligibility Document Environmental Fate and
Effects Science Chapter. June 9, 2007.
http://www.fluoridealert.org/pesticides/docket/EPA-HQ-OPP-2007-0431-0017.pdf
|
|



