|
Return
to Fomesafen Index Page
Activity: Herbicide
(Diphenyl
ether)
Structure:
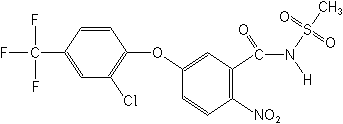
Adverse
Effects:
Blood
Body
Weight Decrease
Bone
Cancer: Possible Human Carcinogen - LIVER
Liver
Stomach
Environmental
• See also: Fomesafen,
sodium
European
Commission: Not allowed to be used as an active ingredient
after July 25, 2003.
US:
In 2003, the US Code of Federal Regulations (CFR) listed
both Fomesafen and the Sodium salt of fomesafen with a tolerance
of 0.05 ppm on Soybean.The
2004 CFR did not list Fomesafen, only the sodium salt. Over
the years EPA has granted several Emergency Exemptions for
the use of "Fomesafen." It is unclear if this
is the sodium salt.
|
Blood
(click on for all fluorinated pesticides)
Decreased
plasma cholesterol and triglycerides and increased liver
weights (reversible at 7 days post-treatment) were observed at
50 mg/kg/day (only dose tested) when administered in the diet
of rats for 4 weeks.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
Body
Weight Decrease (click
on for all fluorinated pesticides)
-- 3. Chronic toxicity.
EPA has not established the RfD for fomesafen. For the purposes
of this tolerance, based upon available chronic toxicity data,
the RfD of 0.0025 mg/kg/day was used. This RfD is based on the
NOEL of 0.25 mg/kg/day from the rat carcinogenicity study. A 100-fold
uncertainty factor was used to calculate this RfD. At the LOEL
of 5.0 mg/kg/day there was liver
toxicity and decreased
body weight.
-- iii. Reproductive toxicity study. In the 2-generation reproductive
toxicity study in rats, the parental (systemic) NOEL was 12.5
mg/kg/ day, based on decreased body weight
and liver necrosis at the LOEL of
50 mg/kg/day. The reproductive and developmental (pup) NOELs were
2.5 mg/kg/day, based on decreased pup body
weight and reduced litter size at the LOEL of 12.5 mg/kg/day.
-- v. Conclusion. Based on the rat reproductive toxicity study
discussed above, the pup LOEL (decreased
body weight and reduced litter size) occurred at levels
below the maternal NOEL and demonstrates post- natal pup toxicity
unrelated to maternal effects. These results are suggestive of
a special sensitivity for infants and children following post-natal
exposure. Therefore, EPA recommends applying an extra 10- fold
uncertainty (safety) factor in the chronic risk analysis. The
low percentage of the RfD occupied by the most highly exposed
child subgroup (4.8% of the RfD; 48% using the extra 10-fold factor)
demonstrates that post-natal risks to infants and children are
low.
Ref: Federal Register. November 19, 1997.
Fomesafen; Pesticide Tolerances for Emergency Exemptions. Final
Rule.
http://www.fluoridealert.org/pesticides/Fomesafen.FR.Nov.19.1997.htm
Bone
(click
on for all fluorinated pesticides)
Acute toxicity. EPA
has selected the developmental NOEL of 7.5 mg/kg/day from the
oral rat developmental toxicity study for the acute dietary endpoint;
at the lowest observed effect level (LOEL) of 50 mg/ kg/day, fetuses
had delayed or partial ossification
and extra ribs.
Ref: Federal Register. July 9, 1997. [OPP-300512;
FRL-5729-5] RIN 2070-AB78 http://www.fluoridealert.org/pesticides/Fomesafen.FR.July.9.1997.htm
Cancer:
Possible Human Carcinogen - LIVER
(click
on for all fluorinated pesticides)
Group
C--Possible Human Carcinogen.
Reviewed 8/ 27/ 86.
Ref:
List of Chemicals Evaluated for Carcinogenic Potential. Science
Information Management Branch, Health Effects Division, Office
of Pesticide Programs, U. S. Environmental Protection Agency.
March 15, 2002.
http://www.biomuncie.org/chemicals_evaluated_for_carcinog.htm
-- Decreased plasma
cholesterol and triglycerides and increased
liver weights (reversible at 7 days post-treatment) were
observed at 50 mg/kg/day (only dose tested) when administered
in the diet of rats for 4 weeks. In a 90-day rat study, dietary
administration of 5 mg/kg/day (LOEL) produced alterations in lipid
metabolism and increases in liver weight. The NOEL was 0.25 mg/kg/day.
In a 26-week dog study, dietary administration of 25 mg/kg/day
(LOEL) produced alterations in lipid metabolism and liver
changes (changes not defined). The NOEL was 1 mg/ kg/day.
Liver toxicity (increased liver masses,
discolored hepatocytes, and pigmented Kupffer cells) was
observed in a 2-year rat feeding study at 50 mg/kg/day (LOEL).
The NOEL was 5 mg/kg/day. Metabolism studies have shown that fomesafen
accumulates in the liver. EPA believes that there is sufficient
evidence for listing fomesafen on EPCRA section 313 pursuant to
EPCRA section 313(d)(2)(B) based on the available hepatic
toxicity data for this chemical.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
-- 4. Carcinogenicity. Fomesafen is classified as a Group
C carcinogen with a Q* of 1.9 x 10-1
(mg/kg/ day)-1. This classification was based on: (i)
Increases in both adenomas and carcinomas at several dose levels
in both sexes of mice; (ii) some evidence of reduced latency for
the time of tumor appearance; (iii) limited evidence of mutagenic
effects; and, (iv) the structural similarity of fomesafen to other
biphenyl ether herbicides which have been shown to be carcinogenic.
-- iii. Cancer risk. Based on exposure levels for drinking water,
as given above, the estimate of cancer risk is 2.7 x 10-6.
This figure is an overestimate, as it was arrived at based on
several very conservative assumptions. Estimates used were calculated
based on data from only one small scale study conducted in NC,
for use of fomesafen on soybeans at a vulnerable site. This represents
a worst case scenario, so is not representative of the ``average''
conditions of use. Additionally, there is language on the product
label warning of the potential of fomesafen
to leach to ground water in vulnerable areas. Vulnerable
areas in this case refers to areas where soils are permeable (sand
and silt loams) and the water table is shallow. The majority of
areas of soybean production, and potential use of fomesafen, will
not likely be vulnerable sites, thus the data used from the one
small scale study greatly overestimates levels which could actually
occur. Further, it is assumed that this exaggerated level will
occur in all drinking water throughout the US, and that each individual
consumes 2 liters of drinking water per day.
-- When considering structural similarities with other chemicals,
fomesafen falls into the class of ``biphenyl ether'' chemical
compounds; this means that this group of chemicals have structural
similarities, including a biphenyl ether group, in common. This
is used as a piece of supporting evidence for the classification
of fomesafen as a Group C carcinogen, since other chemicals of
this group (with similar structure) have been found to be carcinogens.
However, other indications of the carcinogenicity of fomesafen
(i.e., increases of adenomas and carcinomas in a mouse study,
limited evidence of mutagenic effects) were also used in deciding
this cancer classification. At this time, the Agency does not
have sufficient understanding of the structural relationship to
the mechanism of toxicity of these chemicals to conclude that
they may be combined for the purposes of conducting a risk assessment.
Although fomesafen contains some chemical structures in common
with other chemicals that have been found to be carcinogens, EPA
does not yet fully understand the implications of such a relationship,
nor how, or if, these structures relate to the toxicological activity
of the chemical. For the purposes of this tolerance action, therefore,
EPA has not assumed that fomesafen has a common mechanism of toxicity
with other substances.
Ref: Federal Register. November 19, 1997.
Fomesafen; Pesticide Tolerances for Emergency Exemptions. Final
Rule.
http://www.fluoridealert.org/pesticides/Fomesafen.FR.Nov.19.1997.htm
Liver
(click
on for all fluorinated pesticides)
-- 4. Carcinogenicity.
Fomesafen is classified as a Group C carcinogen
with a Q* of 1.9 x 10-1 (mg/kg/ day)-1.
This classification was based on: (i) Increases in both adenomas
and carcinomas at several dose levels in both sexes of mice; (ii)
some evidence of reduced latency for the time of tumor appearance;
(iii) limited evidence of mutagenic effects; and, (iv) the structural
similarity of fomesafen to other biphenyl ether herbicides which
have been shown to be carcinogenic.
-- When considering structural similarities with other chemicals,
fomesafen falls into the class of ``biphenyl ether'' chemical
compounds; this means that this group of chemicals have structural
similarities, including a biphenyl ether group, in common. This
is used as a piece of supporting evidence for the classification
of fomesafen as a Group C carcinogen, since other chemicals of
this group (with similar structure) have been found to be carcinogens.
However, other indications of the carcinogenicity of fomesafen
(i.e., increases of adenomas and carcinomas in a mouse study,
limited evidence of mutagenic effects) were also used in deciding
this cancer classification. At this time, the Agency does
not have sufficient understanding of the structural relationship
to the mechanism of toxicity of these chemicals to conclude that
they may be combined for the purposes of conducting a risk assessment.
Although fomesafen contains some chemical structures in common
with other chemicals that have been found to be carcinogens, EPA
does not yet fully understand the implications of such a relationship,
nor how, or if, these structures relate to the toxicological activity
of the chemical. For the purposes of this tolerance action, therefore,
EPA has not assumed that fomesafen has a common mechanism of toxicity
with other substances.
Ref: Federal Register. November 19, 1997.
Fomesafen; Pesticide Tolerances for Emergency Exemptions. Final
Rule.
http://www.fluoridealert.org/pesticides/Fomesafen.FR.Nov.19.1997.htm
-- Decreased plasma
cholesterol and triglycerides and increased
liver weights (reversible at 7 days post-treatment) were
observed at 50 mg/kg/day (only dose tested) when administered
in the diet of rats for 4 weeks. In a 90-day rat study, dietary
administration of 5 mg/kg/day (LOEL) produced alterations in lipid
metabolism and increases in liver weight. The NOEL was 0.25 mg/kg/day.
In a 26-week dog study, dietary administration of 25 mg/kg/day
(LOEL) produced alterations in lipid metabolism and liver
changes (changes not defined). The NOEL was 1 mg/ kg/day.
Liver toxicity (increased liver masses,
discolored hepatocytes, and pigmented Kupffer cells) was
observed in a 2-year rat feeding study at 50 mg/kg/day (LOEL).
The NOEL was 5 mg/kg/day. Metabolism studies have shown that fomesafen
accumulates in the liver. EPA believes that there is sufficient
evidence for listing fomesafen on EPCRA section 313 pursuant to
EPCRA section 313(d)(2)(B) based on the available hepatic
toxicity data for this chemical.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
-- Classification -- C; possible human carcinogen.
-- Animal Carcinogenicity Data. Limited. Sixty-four Charles River
CD-1 mice/sex/dose group were dosed for 2 years with fomesafen
at 0, 1, 5, 100 and 1000 ppm by dietary incorporation. A double-size
control group was used. At 12 months, 24 mice/sex from the controls
and 12 mice/sex from the treated groups were killed. In
male mice at termination, the incidence of liver adenomas was
significantly increased at 1, 100 and 1000 ppm when compared with
controls. The incidences of liver carcinomas and a combination
of liver adenomas and carcinomas were significantly increased
at 1000 ppm. In the females, the incidence of adenomas was increased
at 100 and 1000 ppm and carcinomas were increased at 1000 ppm
when compared with controls. The incidence
of adenomas and carcinomas combined was significantly increased
at 100 and 1000 ppm. Both sexes, therefore, showed a progression
from benign to malignant tumors with increased dose. Some liver
tumors (adenomas and carcinomas) were apparent at the 52-week
interval kill. There was increased mortality
in the males at 100 and 1000 ppm and in the females at 1000 ppm,
due to liver toxicity forcing termination of the study. The
1000 ppm animals were killed at 79 weeks (males) or 89 weeks (females).
The MTD appeared to be exceeded at 100 ppm in the males and 1000
ppm in the females. The tumor increases occurred at dose levels
of fomesafen that were both below and above the MTD (Huntingdon,
1985).
Ref: US EPA. Fomesafen
(CASRN 72178-02-0). IRIS (Integrated Risk Information System).
-- PubMed Abstract:
Administration of herbicide fomesafen and of fomesafen combined
with one dose of iron to 44 mice during 3 to 14 months caused
hyperplastic and preneoplastic changes in the liver
tissue which had been described in experimental carcinogenesis*
small groups of altered hepatocytes storing glycogen or lipids
and foci of small basophilic liver
cells occurred as early as after 3 months. Altered hepatocytes
were found more frequently in mice getting fomesafen and iron.
Later nodular hyperplasia of liver
cells developed with nodes 3-20 mm in diameter which mostly consisted
of altered hepatocytes with plenty of glycogen. After 12 and 14
month-lasting administration of fomesafen and fomesafen with iron,
the hepatocellular carcinoma was proved in 5 mice. In 4 mice,
the preneoplastic changes in liver
tissue were accompanied by micronodular hyperplasia of liver
cells which did not participate on the development of big nodes
and hepatocellular carcinoma.
Ref: Cesk Patol 1998. Apr;34(2):67-71. [Morphologic
findings in liver tissue in mice after long-term administration
of the herbicide fomesafan] by Chlumska A, Fakan F, Krijt J.
http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query_old?uid=9624829&form=6&db=m&Dopt=b
Stomach
(click
on for all fluorinated pesticides)
-- 2. Short - and intermediate
- term toxicity. EPA has selected the NOEL of 10 mg/kg/day from
the oral rabbit developmental toxicity study for calculation of
short- and intermediate-term margins of exposure (MOEs). At the
LOEL of 40 mg/kg/day, maternal toxicity included stomach
mucosal erosion and death.
Ref: Federal Register. November 19, 1997.
Fomesafen; Pesticide Tolerances for Emergency Exemptions. Final
Rule.
http://www.fluoridealert.org/pesticides/Fomesafen.FR.Nov.19.1997.htm
| Environmental
(click
on for all fluorinated pesticides)
PubMed
abstract: ... In
mesocosms, multiple application of fomesafen, leading to maximal
herbicide concentrations of 60.33 +/- 2.68 microg/L in water,
resulted in reduced number of egg masses and altered glycogen
metabolism in contaminated snails. These
changes, as well as affected steroid-like levels in fomesafen-exposed
snails, support the hypothesis of impaired neuroendocrine
functions.
Ref: Environ
Toxicol Chem 2002 Sep;21(9):1876-88. Nonylphenol polyethoxylate
adjuvant mitigates the reproductive toxicity of fomesafen
on the freshwater snail Lymnaea stagnalis in outdoor experimental
ponds; by Jumel A, Coutellec MA, Cravedi JP, Lagadic L.
--
iii. Cancer risk. Based on exposure levels for drinking
water, as given above, the estimate of cancer risk is 2.7
x 10-6. This figure is an overestimate, as it
was arrived at based on several very conservative assumptions.
Estimates used were calculated based on data from only one
small scale study conducted in NC, for use of fomesafen
on soybeans at a vulnerable site. This represents a worst
case scenario, so is not representative of the ``average''
conditions of use. Additionally, there is language on the
product label warning of the potential
of fomesafen to leach to ground water in vulnerable areas.
Vulnerable areas in this case refers to areas where soils
are permeable (sand and silt loams) and the water table
is shallow. The majority of areas of soybean production,
and potential use of fomesafen, will not likely be vulnerable
sites, thus the data used from the one small scale study
greatly overestimates levels which could actually occur.
Further, it is assumed that this exaggerated level will
occur in all drinking water throughout the US, and that
each individual consumes 2 liters of drinking water per
day.
Ref: Federal Register. November
19, 1997. Fomesafen; Pesticide Tolerances for Emergency
Exemptions. Final Rule.
http://www.fluoridealert.org/pesticides/Fomesafen.FR.Nov.19.1997.htm
Phototoxic
Pesticide.
Light-dependent peroxidizing herbicides
(LDPHs). US EPA identified the the following organofluorine
herbicides Acifluorfen, Azafenidin,
Carfentrazone-ethyl, Flumiclorac-penty, Flumioxazin, Fluthiacet-methyl,
Fomesafen, Lactofen,
Oxadiargyl, Oxadiazon, Oxyfluorfen, Sulfentrazone, Thidiazimin
as phototoxic pesticides
that act by inhibiting protoporphyringen oxidase in the
heme and chlorophyll biosynthetic pathway.
[10 out of the 13 pesticides that EPA identified are organofluorines].
SEE http://www.fluoridealert.org/pesticides/PHOTOTOXICITY.PAGE.htm
Ref: December 11, 2001 - US EPA. Revised
Environmental Fate and Effects Division Preliminary Risk
Assessment for the Oxyfluorfen Reregistration Eligibility
Decision Document (also at: http://www.epa.gov/oppsrrd1/reregistration/oxyfluorfen/oxyefedchap.pdf
).
|
|

