|
Return
to Fluquinconazole Index Page
Activity:
Fungicide (conazole)
Structure:
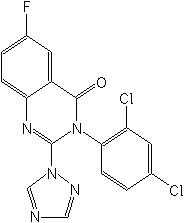
Adverse Effects:
Ataxia
Blood
Body Weight Decrease (emaciation)
Bone
Endocrine: Breast
Endocrine: Pituitary
Endocrine: Thyroid
Fetotoxicity
Kidney
Liver
Environmental: Highly
toxic to fish and algae
•
The acute inhalation LC50 of fluquinconazole in the rat
was 0.75 mg/l (0.83 mg/l in males and 0.51 mg/l in females).
Fluquinconazole is classified under
current EC criteria with R23
Toxic by inhalation.
•
Note from FAN; Labeling according to EC Directives on this
criteria: May
cause cancer by inhalation. May cause heritable genetic
damage. Toxic by inhalation, in contact with skin and if
swallowed. Danger of cumulative effects. Causes severe burns.
Harmful to aquatic organisms, may cause long-term adverse
effects in the aquatic environment.
Ref: Hazardous according
to criteria of Worksafe Australia
http://www.hannainst.com.au/Pro/hi93754b-25mr.htm
|
"8.5.4
Imported Foods. The following are the quantities
of foods that have been imported into Australia in 1999
and 2000. This data are for foods for which reductions and
deletions of MRLs are proposed."
|
| Fluquinconazole |
1999 |
2000 |
| Apple |
33 tonnes |
16 tonnes |
| Pear |
137 tonnes |
11 tonnes
|
Ref:
May 8, 2002.
Final Assessment Report [Inquiry - S.17]. Application A447.
Maximum Residue Limits.
ANZFA. Australia New Zealand Food Authority.
http://www.fluorideaction.org/pesticides/australia.mrls.may.8.2002.pdf
|
Ataxia
(click on for all fluorinated pesticides)
--
Acute oral toxicity. Rat. Groups
of 5 male and 5 female COBS CD Sprague-Dawley rats were each administered
by gavage fluquinconazole (99.6% purity) suspended in a 1% w/v
aqueous methyl-cellulose solution at dose levels of 10, 50 or
250 mg/kg bw. Animals were killes and necropsied after a 14-day
observation period. Five males and 5 females at the 250 mg/kg
bw dose were killed in a moribund condition one day after treatment.
There were no mortalities in either sex at dose levels of ² 50
mg/kg bw. The signs of intoxication prior to death were very severely
reduced activity and reduced muscle tone, severe hunched posture
and slight to moderate ataxia...
The acute oral LD 50 of fluquinconazole in the rat was 112 mg/kg
bw in both sexes. Fluquinconazole is classified with R25 Toxic
if swallowed under current EC criteria.
-- -- Acute oral toxicity. Mouse.
Groups of 5 male and 5 female CRLL:CD (ICR) BR mice were
each administered by gavage fluqinconazole (99.6% purity) dissolved
in a 1% aqueous methyl cellulose solution at nominal dose levels
of 0, 100, 200 or 400 mg/kg bw. The test animals were killed and
necropsied after a 14-day observation period... Signs of intoxication
were observed from 5 h after treatment for up to 5 days in males
at dose levels of ³ 200 mg/kg bw and in females at dose levels
of ³ 100 mg/kg bw. The severity of the signs was dose related
and included reduced activity, laboured respiration, reduced muscle
tone (females only), ataxia, piloerection,
hunched posture, pale extreminities, urogenital soiling (females).
Slight (200 mg/kg bw) to significantly marked (400 mg/kg bw) loss
of body weight was recorded in surviving females after week one.
Body weight gain was normal in males throughout the study and
in females after week one. The acute oral LD 50 of fluquinconazole
in the mouse was 325 mg/kg bw in males and 180 mg/kg bw in females.
-- Acute inhalation toxicity. Groups of 5 male and 5 female Crl:CD
(SD)BR Spraque-Dawley rats were exposed
head-only to a dust aerosol of fluquinconzole (98.7% purity),
at mean analytical concentrations of 0, 0.082, 0.24, 1.08 or 4.56
mg/l for four hours. All surviving animals were killed and necropsied
after a 14-day observation period... signs of toxicity inclding
ataxia, hunched posture and respiratory
distress were observed at the 0.24 mg/l dose for up to 4 days
and at the higher dose levels until death... The acute inhalation
LC50 of fluquinconazole in the rat was 0.75 mg/l (0.83 mg/l in
males and 0.51 mg/l in females). Fluquinconazole is classified
under current EC criteria with R23 Toxic by inhalation.
-- Short Term Toxicity. Oral route. Rat. (b)... Groups of 5 male
and 5 female outbrd albino Sprague-Dawley Crl:COBS CD(SD)BR rats
were each administered fluquinconazole (100% purity) either in
the diet at concentrations of 0 (control), 5, 20, 100 and 400
ppm or by gavage dissolved in 1 % aqueous methlcellulose at a
dose level of 10 mg/kg bw/day for 28 days. Due to mortalities
at the 400 ppm dose level, a supplementary gorups of 5 male and
5 female animals were administered fluquinconazole at a dose level
of 200 ppm... At the 200 ppm dose level 2 males and 5 females
were killed in a moribund condition after 9 and 7 days respectively
of treatment. The signs of toxicity observed showed dose-related
severity. The signs at dose levels of 100 ppm and above included
hunched posture, hypoactivity, ataxia,
reduced mucle tone, piloerection, twitches, urogenital staining,
ptosis and emaciation...
-- Short Term Toxicity. Oral route. Rat.
(c). In a 3-month feeding study, groups of 10 male and 10 female
outbred albino Spraque-Dawley CRL:COBS CD(SD)BR rats were administered
fluquinconazole (>96.2% purity) at concentrations of 0 (controls),
1, 5, 15, or 100 ppm. Additional groups of animals at the 0 (control)
and 100 ppm dose levels were kept on an untreated diet for 4 weeks
in order to invetigate the regressivity of effects. Blood samples
were taken after 6 and 13 weeks of treatment for haematological
and clinical chemistry investigations... Slight signs of intoxication
were observed after 2 weeks in both sexes at the 100 ppm dose
level. The signs included unsteady gait, tremors, hunched posture,
and reduced muscle tone. Lower incidence of reduced activity and
twitches in males, and muscular fibrillation and ataxia
in females were also observed. Body weight gain was significantly
reduced (9%) in males only at the 100 ppm dose level compared
with controls during the treatment period and transient significantly
lower body weights were recorded...
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Blood
(click
on for all fluorinated pesticides)
-- Oral long-term toxicity
and carcinogenicity. 2-year dietary study in rats. groups of 50
male and 50 female outbred albino Sprague-Dawley CRL :COBS CD(SD)BR
rats were administered fluquinconazole (93.2% purity) in the diet
at concentrations of 0 (control), 1, 20 or 100 ppm. Additionally
20 animals/sex/dose designated for the interim-kill received the
test compound at concentrations of 0, 1, 5, 10 or 100 ppm for
12 months... There was a very slight reduction
in red blood cells, haemoglobin and haematocrit in females during
the first 12 months. At 24 months, the mean cell volume, mean
corpuscular haemoglobin (MCH) and haemoglobin values were statistically
significantly lower at the 100 ppm dose level than in controls.
In males, the mean cell volume, mean corpuscular haemoglobin (MCH)
and haemoglobin values were statistically significantly lower
than controls at 12 months only. The
main changes in clinical chemistry were related to serum protein
content...
--
Short Term Toxicity. Oral route. Rat. (b)... Groups of 5 male
and 5 female outbrd albino Sprague-Dawley Crl:COBS CD(SD)BR rats
were each administered fluquinconazole (100% purity) either in
the diet at concentrations of 0 (control), 5, 20, 100 and 400
ppm or by gavage dissolved in 1 % aqueous methlcellulose at a
dose level of 10 mg/kg bw/day for 28 days. Due to mortalities
at the 400 ppm dose level, a supplementary gorups of 5 male and
5 female animals were administered fluquinconazole at a dose level
of 200 ppm... Minimal statistically non-significant differences
were noted in surving male rats at the 200 ppm were reduction
in white blood cells, reduction in lymphocytes and increased mean
cell haemoglobin concentration. Changes in clinical chemistry
parameters were related primarly to plasma proteins...
-- Short Term Toxicity. Oral route. Rat. (c). In a 3-month feeding
study, groups of 10 male and 10 female outbred albino Spraque-Dawley
CRL:COBS CD(SD)BR rats were administered fluquinconazole (>96.2%
purity) at concentrations of 0 (controls), 1, 5, 15, or 100 ppm.
Additional groups of animals at the 0 (control) and 100 ppm dose
levels were kept on an untreated diet for 4 weeks in order to
invetigate the regressivity of effects. Blood samples were taken
after 6 and 13 weeks of treatment for haematological and clinical
chemistry investigations... Haematology,
clinical chemistry and urinalysis parameters showed
incidences of statistically significant changes which were
considred to be of limited toxicological significance because
they were either not dose-related or were reported to be withing
the range of historical data.
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Body
Weight Decrease
(click
on for all fluorinated pesticides)
-- Oral long-term toxicity
and carcinogenicity. 2-year dietary study in rats. groups of 50
male and 50 female outbred albino Sprague-Dawley CRL :COBS CD(SD)BR
rats were administered fluquinconazole (93.2% purity) in the diet
at concentrations of 0 (control), 1, 20 or 100 ppm. Additionally
20 animals/sex/dose designated for the interim-kill received the
test compound at concentrations of 0, 1, 5, 10 or 100 ppm for
12 months... Mortalities over the 24-month period were 24/50,
28/50, 28/50 and 31/50 in males and in females 33/50, 36/50 and
39/50 at dose levels of 0, 1, 20 and 100 ppm... Post-mortem examinations
showed the most probable cause of deaths in males to be pituitary
tumors followed by chronic progressive nephropathy and urinary
tract infections. In females mammmary tumors followed by pituitary
tumors were considered the most probable cause of morbidity...
Feed consumption was very slightly increased at the 100 ppm dose
level (7% in males and 15% in females over weeks 1-104) compared
to controls. Body weight gain was
during weeks 0-55 reduced in treated females (9-12%) compared
with controls but was significantly reduced
during weeks 55-103 in males (13% at 10 ppm and 68% at 100 ppm)
and in females (40% at 100 ppm dose level)...
--
Short Term Toxicity. Oral route. Rat. (b)... Groups of 5 male
and 5 female outbrd albino Sprague-Dawley Crl:COBS CD(SD)BR rats
were each administered fluquinconazole (100% purity) either in
the diet at concentrations of 0 (control), 5, 20, 100 and 400
ppm or by gavage dissolved in 1 % aqueous methlcellulose at a
dose level of 10 mg/kg bw/day for 28 days. Due to mortalities
at the 400 ppm dose level, a supplementary gorups of 5 male and
5 female animals were administered fluquinconazole at a dose level
of 200 ppm... At the 200 ppm dose level 2 males and 5 females
were killed in a moribund condition after 9 and 7 days respectively
of treatment. The signs of toxicity observed showed dose-related
severity. The signs at dose levels of 100 ppm and above included
hunched posture, hypoactivity, ataxia, reduced mucle tone, piloerection,
twitches, urogenital staining, ptosis and emaciation...
Reductions in body weight gain occurred
in males only at 100 ppm (17%) and 10 mg/kg bw/day (16%)
ppm compared to controls. The reduction
in body weight gain in the surviving males at the 200 ppm dose
level was 39% compared with that of the control over the
treatment period...
-- Short Term Toxicity. Oral route. Rat. (c). In a 3-month feeding
study, groups of 10 male and 10 female outbred albino Spraque-Dawley
CRL:COBS CD(SD)BR rats were administered fluquinconazole (>96.2%
purity) at concentrations of 0 (controls), 1, 5, 15, or 100 ppm.
Additional groups of animals at the 0 (control) and 100 ppm dose
levels were kept on an untreated diet for 4 weeks in order to
invetigate the regressivity of effects. Blood samples were taken
after 6 and 13 weeks of treatment for haematological and clinical
chemistry investigations... Slight signs of intoxication were
observed after 2 weeks in both sexes at the 100 ppm dose level.
The signs included unsteady gait, tremors, hunched posture, and
reduced muscle tone. Lower incidence of reduced activity and twitches
in males, and muscular fibrillation and ataxia in females were
also observed. Body weight gain was significantly
reduced (9%) in males only at the 100 ppm dose level compared
with controls during the treatment period and transient significantly
lower body weights were recorded...
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Bone
(click on for all fluorinated pesticides)
-- Developmental toxicity
studies. (a) Oral teratology study in rats. In a study (1992),
groups of 30 mated outbred albino Sprague-Dawley CRL:COBS CD(SD)BR
rats were administered by gavage fluqinconazole (96% purity) in
a 1% w/v aqueous methylcellulose solution at concentrations of
0, 0.4, 2 and 20 mg/kg bw/day (based on a range-finding study)
from day 6 to 15 of presumed gestation... An increase
in abnormal sternebrae was seen in the low dose group (27%)
and the high dose group (31%, p<0.05) but not at the mid dose
group (15%) when compared to controls (13%) - as the incidence,
of this relatively common anomaly, in the low dose group is not
statistically significant and not party of a dose response it
is considered to be of no biological significance. Fluquinconazole
was clearly maternally toxic, producing abortion and mortality
at 8 mg/kg bw/day. There was evidence of
mild fetotoxicity (abnormal sternebrae) but not teratogenicity
at 9 mg/kg bw/day. The NOAEL for maternal and fetotoxicity was
2 mg/kg bw/day based on increased incidence of abortions in dams
and increased mortality.
-- In the rabbit the NOEL for maternal and fetal toxicity was
2 mg/kg bw/day. Fluquinconazole was not teratogenic in the rabbit
in the presence of maternal toxicity. Fluquinconazole was clearly
maternally toxic, producing abortion and mortality at 8 mg/kg
bw/day. There was evidence of mild fetotoxicity
(abnormal sternebrae) but not teratogenicity at 8 mg/kg
bw/day.
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Endocrine:
Breast (click
on for all fluorinated pesticides)
-- Oral long-term toxicity
and carcinogenicity. 2-year dietary study in rats. groups of 50
male and 50 female outbred albino Sprague-Dawley CRL :COBS CD(SD)BR
rats were administered fluquinconazole (93.2% purity) in the diet
at concentrations of 0 (control), 1, 20 or 100 ppm. Additionally
20 animals/sex/dose designated for the interim-kill received the
test compound at concentrations of 0, 1, 5, 10 or 100 ppm for
12 months... Mortalities over the 24-month period were 24/50,
28/50, 28/50 and 31/50 in males and in females 33/50, 36/50 and
39/50 at dose levels of 0, 1, 20 and 100 ppm... Post-mortem examinations
showed the most probable cause of deaths in males to
be pituitary tumors followed by chronic
progressive nephropathy and
urinary tract infections. In females mammmary
tumors followed by pituitary tumors
were considered the most probable cause of morbidity... In the
thyroid gland a significantly higher incidence in follicular cell
tumors were noted in the 100 ppm dose level in both sexes. Historical
tumor incidences provided showed that the slight statistically
non-significant increase in thyroid follicular cell tumors at
the lowest and intermediate dose levels were at the upper limit
of the historical control range and therefore in the absence
of a dose response they were considered to be not of toxicological
significance...
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Endocrine:
Pituitary
(click on for all fluorinated pesticides)
-- Oral long-term toxicity
and carcinogenicity. 2-year dietary study in rats. groups of 50
male and 50 female outbred albino Sprague-Dawley CRL :COBS CD(SD)BR
rats were administered fluquinconazole (93.2% purity) in the diet
at concentrations of 0 (control), 1, 20 or 100 ppm. Additionally
20 animals/sex/dose designated for the interim-kill received the
test compound at concentrations of 0, 1, 5, 10 or 100 ppm for
12 months... Mortalities over the 24-month period were 24/50,
28/50, 28/50 and 31/50 in males and in females 33/50, 36/50 and
39/50 at dose levels of 0, 1, 20 and 100 ppm... Post-mortem examinations
showed the most probable cause of deaths in males to be pituitary
tumors followed by chronic progressive
nephropathy and
urinary tract infections. In females mammmary
tumors followed by pituitary tumors
were considered the most probable cause of morbidity... In the
thyroid gland a significantly higher incidence in follicular
cell tumors were noted in the 100 ppm dose level in both
sexes. Historical tumor incidences provided showed that the slight
statistically non-significant increase in thyroid follicular cell
tumors at the lowest and intermediate dose levels were
at the upper limit of the historical control range and therefore
in the absence of a dose response they were considered to be not
of toxicological significance...
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK.Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Endocrine:
Thyroid
(click
on for all fluorinated pesticides)
-- Groups of 40 male
Sprague Dawley rats were fed a diet containing fluquinaconazole
at concentrations of 0 (control) or 100 ppm for 14 days. Twenty
rats per dose group were killed and necropsied on day 15. The
remaining animals were kept for a further 14 days on a treatment-free
diet then killed and necropsied... Hormone assays for thyroxine
(T4), triiodothyronine (T3), thyrotropin (TSH) and reverse triiodothyronine
(RT3) were conducted but the details of the assay methods were
not provided... Histopathology of the thyroid was reported and
focussed on 3 parameters; colloid depletion, follicular cell hypertrophy
and follicular cell hyperplasia... Histopathology
of the thyroid showed the most distinctive finding to be the increased
incidences of follicular epithelial hyperplasia of the thyroid
(11/20) compared with 0/20 in controls at day 15). An increase
in the incidence of severe folicular hypertrophy and severe or
very severe colloid depletion was considered to be also indicative
of increase in thryoidtoxicity in fluquinconazole-treated animals.
Severe to very severe colloidal depletion (5/20 animals compared
with 1/20 in controls), severe follicular cell hypertrophy (3/20
compared wtih 0/20 in controls) and slight to moderate follicular
epithelial hyperplasia (4/20 compared with 1/20 in controls) was
present in the thyroid at the end of the recovery period. In conclusion,
administration of fluquinconazole to male rats resulted in changes
in thyroid hormone homeostasis manifested by histopathological
changes int he thyroid... (DP 47301)
-- Oral long-term toxicity and carcinogenicity. 2-year dietary
study in rats. groups of 50 male and 50 female outbred albino
Sprague-Dawley CRL :COBS CD(SD)BR rats were administered fluquinconazole
(93.2% purity) in the diet at concentrations of 0 (control), 1,
20 or 100 ppm. Additionally 20 animals/sex/dose designated for
the interim-kill received the test compound at concentrations
of 0, 1, 5, 10 or 100 ppm for 12 months... In the thyroid
gland a significantly higher incidence in follicular
cell tumors were noted in the 100 ppm dose level in both
sexes. Historical tumor incidences provided showed that the slight
statistically non-significant increase in thyroid
follicular cell tumors at the lowest and intermediate dose
levels were at the upper limit of the historical control range
and therefore in the absence of a dose response they were considered
to be not of toxicological significance.
-- The rat was the most sensitive species
to fluquinconazole compared with the dog and mouse. The main target
organs were the liver, kidney and thyroid... For the
thyroid tumors in the rat, a correlation between induction
of liver UDGPT and imbalances in thyroid hormone (increase in
TSH and reduction in T4) and histopathological changes in the
thyroid was demonstrated. Disturbance of thyroid hormone homeostasis
is a well recognised mechanism for the induction of thyroid tumours.
Futher this is considered to be secondary to the observed liver
enzme induction. A clear NOEL for thyroid
tumors and the induction of thryoid effects was established.
--
Acute inhalation toxicity. Groups of 5 male and 5 female Crl:CD
(SD)BR Spraque-Dawley rats were exposed head-only to a dust aerosol
of fluquinconzole (98.7% purity), at mean analytical concentrations
of 0, 0.082, 0.24, 1.08 or 4.56 mg/l for four hours. All surviving
animals were killed and necropsied after a 14-day observation
period... signs of toxicity inclding ataxia, hunched posture and
respiratory distress were observed at the 0.24 mg/l dose for up
to 4 days and at the higher dose levels until death... In the
thyroid, the incidence and severity
of thyroid effects including colloid depletion, follicular cell
hyperplasia and hypertrophy did not show a clear treatment-relationship
(statistically significant compared with
controls at 100 and 200 ppm but not at 400 ppm and 10 mg/kg
bw/day dose levels). The NOEl was 5 ppm (0.45 mg/kg bw/day) based
on increased liver and kidney weights and histopathological changes
in the liver, kidney and thyroid
at the higher dose level of 20 ppm... The
liver, thyroid and kidneys were noted to be the target organs
of the test compound and significant changes in these organs
indicative of significant hepatic enzyme induction were considered
to be relevant for risk assessment.
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Fetotoxicity
(click
on for all fluorinated pesticides)
- Developmental toxicity
studies. (a) Oral teratology study in rats.
In a study (1992), groups of 30 mated outbred albino Sprague-Dawley
CRL:COBS CD(SD)BR rats were administered by gavage fluqinconazole
(96% purity) in a 1% w/v aqueous methylcellulose solution at concentrations
of 0, 0.4, 2 and 20 mg/kg bw/day (based on a range-finding study)
from day 6 to 15 of presumed gestation... An increase
in abnormal sternebrae was seen in the low dose group (27%)
and the high dose group (31%, p<0.05) but not at the mid dose
group (15%) when compared to controls (13%) - as the incidence,
of this relatively common anomaly, in the low dose group is not
statistically significant and not party of a dose response it
is considered to be of no biological significance.
Fluquinconazole was clearly maternally toxic, producing abortion
and mortality at 8 mg/kg bw/day. There
was evidence of mild fetotoxicity (abnormal sternebrae)
but not teratogenicity at 9 mg/kg bw/day. The
NOAEL for maternal and fetotoxicity was 2 mg/kg bw/day based on
increased incidence of abortions in dams and increased mortality.
-- In the rabbit the NOEL for maternal
and fetal toxicity was 2 mg/kg bw/day. Fluquinconazole was not
teratogenic in the rabbit in the presence of maternal toxicity.
Fluquinconazole was clearly maternally toxic, producing abortion
and mortality at 8 mg/kg bw/day. There was
evidence of mild fetotoxicity (abnormal sternebrae) but not teratogenicity
at 8 mg/kg bw/day.
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Kidney
(click
on for all fluorinated pesticides)
-- Oral long-term toxicity
and carcinogenicity. 2-year dietary study in rats. groups of 50
male and 50 female outbred albino Sprague-Dawley CRL :COBS CD(SD)BR
rats were administered fluquinconazole (93.2% purity) in the diet
at concentrations of 0 (control), 1, 20 or 100 ppm. Additionally
20 animals/sex/dose designated for the interim-kill received the
test compound at concentrations of 0, 1, 5, 10 or 100 ppm for
12 months... At the interim-kill, organ weights showed moderate
significant treatment-realted increase in the absolute liver and
kidney weights in males and females at the 100 ppm dose
level compared with controls. The relative
liver and kidney weights were significantly
increased in males at the 100 ppm dose level and in females at
³ 10 ppm. A significant increase in the incidence of hyaline droplet
deposition in the proximal tubular epithelium occurred in the
kidneys of males (15/20 compared with 6/20 controls) at
the 100 ppm dose level. Minimal to moderate hypertrophy of centrilobular
hepatocytes was observed in the liver of all top dose animals
and at the 10 ppm dose level (10/20 males and 3/20 females). In
the thyroid, an increase in the follicular epithelial height in
both sexes was noted at the 100 ppm dose level. At the terminal-kill,
the absoloute and relative liver and kidney
weights of males and females were slightly to moderately
increased at 100 ppm dose level...
-- Kidney effects were observed in
the rat and mouse and consisted of increases in organ weight and
histopathological changes including hyaline droplet nephropathy,
focal tubular hyperplasia, increases in the incidence of small
and large eosinophilic inclusions, and increase in the incidence
and severity of chronic progressive nephropathy. There is no conclusive
mechanism for the observed kidney effects.
-- Short Term Toxicity. Oral route. Rat. (b)... Groups of 5 male
and 5 female outbrd albino Sprague-Dawley Crl:COBS CD(SD)BR rats
were each administered fluquinconazole (100% purity) either in
the diet at concentrations of 0 (control), 5, 20, 100 and 400
ppm or by gavage dissolved in 1 % aqueous methlcellulose at a
dose level of 10 mg/kg bw/day for 28 days. Due to mortalities
at the 400 ppm dose level, a supplementary gorups of 5 male and
5 female animals were administered fluquinconazole at a dose level
of 200 ppm... Organ weights showed a dose-related increase in
the relative liver weight at dose levels of ³ 20 ppm in males
(15-44%) and at 100 ppm and 10 mg/kg bw/day dose levels (25-27%).
The relative kidney weight was increased
in males only (11-21%) compared with controls at dose levels
of ³ 20 ppm. Gross examination at necropsy revealed accentuation
of the lobular pattern of the liver at dose levels of ³ 5 ppm
in females and at ³ 20 ppm in males. Pale
kidneys were observed in males at dose levels of ² 5 ppm...
Histopathology showed significant treatment-related centrilobular
hepatocyte hypertrophy in males and females at dose levels of
³ 100 ppm. In the kidney, slight to moderate
hyaline droplet mephropathy was observed in males at ³ 20 ppm...
The liver, thyroid and kidneys were noted
to be the target organs of the test compound and significant
changes in these organs indicative of significant hepatic enzyme
induction were considered to be relevant for risk assessment.
-- Short Term Toxicity. Oral route. Rat. (c). In a 3-month feeding
study, groups of 10 male and 10 female outbred albino Spraque-Dawley
CRL:COBS CD(SD)BR rats were administered fluquinconazole (>96.2%
purity) at concentrations of 0 (controls), 1, 5, 15, or 100 ppm.
Additional groups of animals at the 0 (control) and 100 ppm dose
levels were kept on an untreated diet for 4 weeks in order to
invetigate the regressivity of effects. Blood samples were taken
after 6 and 13 weeks of treatment for haematological and clinical
chemistry investigations... Slight signs of intoxication were
observed after 2 weeks in both sexes at the 100 ppm dose level.
The signs included unsteady gait, tremors, hunched posture, and
reduced muscle tone. Lower incidence of reduced activity and twitches
in males, and muscular fibrillation and ataxia
in females were also observed. Body weight gain was significantly
reduced (9%) in males only at the 100 ppm dose level compared
with controls during the treatment period and transient significantly
lower body weights were recorded... Haematology, clinical chemistry
and urinalysis parameters showed incidences of statistically significant
changes which were considred to be of limited toxicological significance
because they were either not dose-related or were reported to
be withing the range of historical data. There was an increase
in the absolute liver (35%) and adrenal (36.4%) weights in females
at the 100 ppm dose level. The relative
kidney (14%) and liver (21%) weights in males and the
relative kidney (6.9%) and adrenal (30%) weights in females
were significantly increased at the
100 ppm dose level. In females, the relative liver weight was
increased at dose levels of 15 ppm (14%) and 100 ppm (32%). At
the end of the withdrawal period, the relative
kidney weight in males and the relative liver weight in females
were still siginficantly elevated compared with controls...
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
Liver
(click
on for all fluorinated pesticides)
-- Oral long-term toxicity
and carcinogenicity. 2-year dietary study in rats. groups of 50
male and 50 female outbred albino Sprague-Dawley CRL :COBS CD(SD)BR
rats were administered fluquinconazole (93.2% purity) in the diet
at concentrations of 0 (control), 1, 20 or 100 ppm. Additionally
20 animals/sex/dose designated for the interim-kill received the
test compound at concentrations of 0, 1, 5, 10 or 100 ppm for
12 months... Mortalities over the 24-month period were 24/50,
28/50, 28/50 and 31/50 in males and in females 33/50, 36/50 and
39/50 at dose levels of 0, 1, 20 and 100 ppm... Post-mortem examinations
showed the most probable cause of deaths in males to be pituitary
tumors followed by chronic progressive
nephropathy and urinary tract infections.
In females mammmary tumors followed by pituitary tumors were considered
the most probable cause of morbidity... At the interim-kill,
organ weights showed moderate significant treatment-related increase
in the absolute liver and kidney
weights in males and females at the 100 ppm dose level compared
with controls. The relative liver
and kidney weights were significantly increased
in males at the 100 ppm dose level and in females at ³ 10 ppm.
A significant increase in the incidence of hyaline droplet deposition
in the proximal tubular epithelium occurred in the kidneys of
males (15/20 compared with 6/20 controls) at the 100 ppm dose
level. Minimal to moderate hypertrophy of
centrilobular hepatocytes was observed in the liver
of all top dose animals and at the 10 ppm dose level (10/20
males and 3/20 females). In the thyroid, an increase int he follicular
epithelial height in both sexes was noted at the 100 ppm dose
level. At the terminal-kill, the absoloute and relative liver
and kidney weights of males and females were slightly to
moderately increased at 100 ppm dose level. Necropsy revealed
slightly increased incidence of liver masses
in top dose females (0/50, 0/50 and 6/50 at dose levels of 0.
1, 10 and 100 ppm respectively). Microscopic findings at 24 months
were slight increases in the incidence and severity of chronic
progressive nephropathy in males and females at the 100
ppm dose level. Slight to severe centrilobular
cellular hypertrophy, bile duct hyperplasia (51/100 compared
with 21/100 for controls in both sexes) and significant
increase in the incidence of malignant liver tumors (8/50
compared with 0/50 in males) in females occurred at the top dose
level...
-- For liver tumours, no clearcut
identifiable preneoplastic lesions were identified (No data requirement
for this to be demonstrated has previously been requested). Liver
tumors in both sexes at 100 ppm were associated with increased
liver weight, increased incidence and severity of hepatocyte hypertrophy
(both of which were observed in shorter term studies and responded
to changes in dose), and bile duct hyperplasia and a slight increase
in eosinophilic foci in females seen in the chronic study of the
rat at 24 months only. Clear NOELs for liver tumors and the induction
of associated liver effects were determined in rats and mice.
-- Acute inhalation toxicity. Groups of 5 male and 5 female Crl:CD
(SD)BR Spraque-Dawley rats were exposed head-only to a dust aerosol
of fluquinconzole (98.7% purity), at mean analytical concentrations
of 0, 0.082, 0.24, 1.08 or 4.56 mg/l for four hours. All surviving
animals were killed and necropsied after a 14-day observation
period... signs of toxicity inclding ataxia,
hunched posture and respiratory distress were observed at the
0.24 mg/l dose for up to 4 days and at the higher dose levels
until death... The acute inhalation LC50 of fluquinconazole in
the rat was 0.75 mg/l (0.83 mg/l in males and 0.51 mg/l in females).
Fluquinconazole is classified under current EC criteria with R23
Toxic by inhalation.
-- Short Term Toxicity. Oral route. Rat. (b)... Groups of 5 male
and 5 female outbred albino Sprague-Dawley Crl:COBS CD(SD)BR rats
were each administered fluquinconazole (100% purity) either in
the diet at concentrations of 0 (control), 5, 20, 100 and 400
ppm or by gavage dissolved in 1 % aqueous methlcellulose at a
dose level of 10 mg/kg bw/day for 28 days. Due to mortalities
at the 400 ppm dose level, a supplementary gorups of 5 male and
5 female animals were administered fluquinconazole at a dose level
of 200 ppm...Organ weights showed a dose-related increase in the
relative liver weight at dose levels
of ³ 20 ppm in males (15-44%) and at 100 ppm and 10 mg/kg bw/day
dose levels (25-27%). The relative kidney weight was increased
in males only (11-21%) compared with controls at dose levels of
³ 20 ppm. Gross examination at necropsy revealed accentuation
of the lobular pattern of the liver
at dose levels of ³ 5 ppm in females and at ³ 20 ppm in males.
Pale kidneys were observed in males at dose levels of ² 5 ppm...
Histopathology showed significant treatment-related
centrilobular hepatocyte hypertrophy in males and females
at dose levels of ³ 100 ppm. In the kidney, slight to moderate
hyaline droplet mephropathy was observed in males at ³ 20 ppm.
In the thyroid, the incidence and severity of thyroid effects
including colloid depletion, follicular cell hyperplasia and hypertrophy
did not show a clear treatment-relationship (statistically significant
compared with controls at 100 and 200 ppm but not at 400 ppm and
10 mg/kg bw/day dose levels). The NOEl was 5 ppm (0.45 mg/kg bw/day)
based on increased liver and kidney
weights and histopathological
changes in the liver, kidney and thyroid at the higher
dose level of 20 ppm... The liver, thyroid
and kidneys were noted to be the target organs of the test compound
and significant changes in these organs indicative of significant
hepatic enzyme induction were considered to be relevant for risk
assessment.
-- Haematology, clinical chemistry and urinalysis parameters showed
incidences of statistically significant changes which were considred
to be of limited toxicological significance because they were
eithe not dose-related or were reported to be withing the range
of historical data. There was an increase in the absolute
liver (35%) and adrenal (36.4%) weights in females at the
100 ppm dose level. The relative kidney (14%) and
liver (21%) weights in males and the relative kidney (6.9%)
and adrenal (30%) weights in females were significantly increased
at the 100 ppm dose level. In females, the relative liver
weight was increased at dose levels of 15 ppm (14%) and 100 ppm
(32%). At the end of the withdrawal period, the relative
kidney weight in males and the relative liver
weight in females were still siginficantly elevated compared with
controls. Gross examination of organs revealed accentuated
lobular pattern of the liver in males and females at the
15 ppm (on male only) and 100 ppm (3 males and 6 females) dose
levels but a significant reduction in the
incidence of lobulation of the liver (1 male only) was
observed at the end of the withdrawal period.
Ref: Evaluation on: Fluquinconazole. May
1999. No. 184. Evaluation of Fully Approved or Provisionally Approved
Products. Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings Pool, 3 Peasholme
Green, York YO1 7 PX, UK. Available online:
http://www.pesticides.gov.uk/citizen/evaluations/evallist.htm
|
Environmental
(click
on for all fluorinated pesticides)
Environmental
/ Ecological Effects: Fluquinconazole (page 5)
Fish Toxicity:
LC50 (96 hr) rainbow trout 1.9 mg/L;
bluegill sungish 1.34 mg/L; carp 1.9 mg/L
Other:
LC50 (48 hr) Daphnia >5
mg/L. ErC50 (96 hr) algae 0.046 mg/L; EbC50 (96 hr) algae
0.014 mg/L
Ref: September 2003.
Material Safety Data Sheet for Vision 250 SC Fungicide.
Bayer CropScience (Australia).
Labeling according to EC Directives on this criteria:
...
Harmful to aquatic organisms, may cause long-term adverse
effects in the aquatic environment.
Ref: Hazardous according to
criteria of Worksafe Australia
http://www.hannainst.com.au/Pro/hi93754b-25mr.htm
|
|

