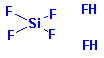|
Return
to Fluosilicic Acid Index Page
Activity:
Wood
Preservative
Structure:
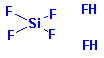
Adverse
Effects:
Bone
Dermal
Eye
Gastrointestinal tract
The
major use
of sodium hexafluorosilicate and fluorosilicic
acid is as fluoridation agents for drinking water.
Both chemicals are also used as a chemical intermediate
(raw material) for aluminum trifluoride, cryolite (Na3AlF6),
silicon tetrafluoride, and other fluorosilicates and have
found applications in commercial laundry. Fluorosilicic
acid is used in the tanning of animal hides and skins, in
ceramics and glass, in technical paints, in oil well acidizing,
in the manufacture of hydrogen fluoride, for the sterilization
of equipment (e.g., in brewing and bottling establishments
and for copper and brass vehicles), and in electroplating.
It is also employed as an impregnating ingredient to preserve
wood and harden masonry and for
the removal of mold as well as rust and stain in
textiles.
• Fluorosilicic acid
is mainly produced as a byproduct of the manufacture of
phosphate fertilizers... In
the manufacture of phosphate fertilizer in Central Florida,
fluorides and radionuclides
(radium and uranium) are released as toxic pollutants.
During the acidulation process, radon gas can be released
and carried into the fluorosilicic acid, while polonium
can be captured during the
scrubbing process and combined with fluoride.
Ref: Toxicological Summary
for Sodium Hexafluorosilicate [CASRN 16893-85-9] and Fluorosilicic
Acid [CASRN 16961-83-4]. Review of Toxicological Literature.
October 2001. Prepared for Scott Masten, Ph.D. National
Institute of Environmental Health Sciences.
http://ntp-server.niehs.nih.gov/htdocs/Chem_Background/ExSumPDF/Fluorosilicates.pdf
|
Bone
(click on for all fluorinated pesticides)
-- The effects of long-term
exposure to fluorosilicic acid are changes in
bone, corrosivity of the mucous membranes (e.g., ulceration
of the nose, throat, and bronchial tubes), coughing, shock, pulmonary
edema, fluorosis, coma, and even death (LCI, Ltd., undated-a).
In a study of 50 workers engaged for approximately 30 years in
the production of phosphate fertilizers, the concentration of
gaseous fluoride (hydrogen fluoride, silicon tetrafluoride, and
fluorosilicic acid) ranged from 0.04 to 0.17 mg/m 3 . Nine
workers had increased bone densities (Fabbri et al., 1978;
cited by HSDB, 2000a).
Ref: Sodium Hexafluorosilicate [CASRN 16893-85-9]
and Fluorosilicic Acid [CASRN 16961-83-4]. Review of Toxicological
Literature. October 2001. Prepared for Scott Masten, Ph.D. National
Institute of Environmental Health Sciences P.O. Box 12233 Research
Triangle Park, North Carolina 27709 Contract No. N01-ES-65402.
Submitted by Karen E. Haneke, M.S. (Principal Investigator) Bonnie
L. Carson, M.S. (Co-Principal Investigator) Integrated Laboratory
Systems P.O. Box 13501 Research Triangle Park, North Carolina
27709.
http://www.fluoridealert.org/pesticides/Fluorosilicates.NIH.2001.pdf
Dermal
(click on for all fluorinated
pesticides)
-- Rats, guinea pigs,
and swine tested as a group; no other data were provided, percutaneous;
The intact skin was not affected. When areas were injured before
application of the acid, necrosis, continuously spreading, occurred
in the deeper regions. Hypocellular necrosis,
consisting of sharp leukocyte demarcations, and edema
up to the subcutis were observed. Alhassan and Zink (1982; cited
by HSDB, 2000a)
-- Rabbits, New Zealand; 0.5 mL (4 mol) to the intact and abraded
skin for 1, 24, or 72 h Severe erythema
and edema were observed, indicating the material to be
a primary irritant. Rhone- Poulenc Inc. (1971)
Ref: Review of Toxicological Literature.
October 2001. Sodium Hexafluorosilicate [CASRN 16893-85-9] and
Fluorosilicic Acid [CASRN 16961-83-4]. Prepared for Scott Masten,
Ph.D. National Institute of Environmental Health Sciences P.O.
Box 12233 Research Triangle Park, North Carolina 27709. Contract
No. N01-ES-65402. Submitted by Karen E. Haneke, M.S. (Principal
Investigator) Bonnie L. Carson, M.S. (Co-Principal Investigator)
Integrated Laboratory Systems P.O. Box 13501 Research Triangle
Park, North Carolina 27709.
http://www.fluoridealert.org/pesticides/Fluorosilicates.NIH.2001.pdf
Eye
(click
on for all fluorinated pesticides)
-- Rabbits, New Zealand,
fluorosilicic acid (~ 23%, neat), purity n. p. instillation; 0.1
mL (0.8 mol) into the left eye. Eyes were observed at 24, 48,
and 72 h following treatment. Severe and
permanent corneal opacity with scar tissue occurred. Rhone-
Poulenc Inc. (1971)
Ref: Review of Toxicological Literature.
October 2001. Sodium Hexafluorosilicate [CASRN 16893-85-9] and
Fluorosilicic Acid [CASRN 16961-83-4]. Prepared for Scott Masten,
Ph.D. National Institute of Environmental Health Sciences P.O.
Box 12233 Research Triangle Park, North Carolina 27709. Contract
No. N01-ES-65402. Submitted by Karen E. Haneke, M.S. (Principal
Investigator) Bonnie L. Carson, M.S. (Co-Principal
Investigator) Integrated Laboratory Systems P.O. Box 13501 Research
Triangle Park, North Carolina 27709.
http://www.fluoridealert.org/pesticides/Fluorosilicates.NIH.2001.pdf
Gastrointestinal
tract
(click on for all fluorinated
pesticides)
-- Rats, oral; 430
mg/ kg (LD 50 ; 2.98 mmol/ kg); Somnolence and/ or general depressed
activity was observed. RTECS* (2000)
-- Rats, Sprague- Dawley albino, oral (via stomach tube); single
doses of 215, 464, 1000, and 2100 mg/ kg (1.49, 3.22, 6.939, and
14.57 mmol/ kg) dissolved in water. Animals were observed for
14 days and then necropsied. With 464 mg/ kg, 3 out of 5 rats
died; at ¥ 1000 mg/ kg, 100% mortality was observed. At ¥ 464
mg/ kg, acute depression was observed. Necropsy showed that animals
in the low- dose group were "grossly normal" and that dead rats
had massive hemorrhages in the entire gastrointestinal
tract. Rhone- Poulenc Inc. (1971)
Ref: Review of Toxicological Literature.
October 2001. Sodium Hexafluorosilicate [CASRN 16893-85-9] and
Fluorosilicic Acid [CASRN 16961-83-4]. Prepared for Scott Masten,
Ph.D. National Institute of Environmental Health Sciences P.O.
Box 12233 Research Triangle Park, North Carolina 27709. Contract
No. N01-ES-65402. Submitted by Karen E. Haneke, M.S. (Principal
Investigator) Bonnie L. Carson, M.S. (Co-Principal Investigator)
Integrated Laboratory Systems P.O. Box 13501 Research Triangle
Park, North Carolina 27709.
http://www.fluoridealert.org/pesticides/Fluorosilicates.NIH.2001.pdf
|