|
Return
to Fluometuron Index Page
Return
to Abstracts
Activity: Herbicide
(phenylurea)
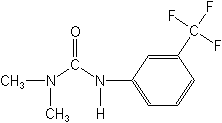
Structure:
Adverse Effects:
Ataxia
Blood
Body Weight Decrease
Bone
Brain
Cancer: Possible Human Carcinogen:
Lung, Lymphocytic
lymphomas, (Liver?)
CNS
Endocrine: Pituitary
Endocrine: Testicular
Eye
Kidney
Leukemia
Liver
Lung
Reproductiv/Developmental
Spleen
Environmental
The
significant cancer risk contributors have been identified
as drinking water (direct and indirect, all sources), and
several rotational crops with wheat (flour), soybean (oil),
and rice (white) having the highest contributions.
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk
Assessment for Phase III of the Reregistration Eligibility
Decision. Docket Identification Number: OPP-2004-0372-0008. (page
7)
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
Twelve
of the 26 most widely used pesticides in the U.S. are classified
as possible or probable carcinogens by the EPA based on
studies of laboratory animals, with an annual use that totals
380 million pounds. (atrazine
(C=possible), metolachlor (C), metarn sodium (B2=probable),
dichloropropene (B2), cyanazine (C), pendimethalin (C),
trifluralin (C), acetochlor (B2), chlorothalonil (likely),
mancozeb (B2), fluometuron
(C), and parathion (C)... (Journal
of Pesticide Reform, Summer 1999, at 5).
Ref: Testimony of the Maine
Organic Farmers and Gardeners Association In support of
L.D. 1540. March 29, 2001.
http://www.mofga.org/ge_pesticide_test.html
--
Note from FAN: Trifluralin, cited above, is another organofluorine
pesticide.
|
Ataxia
(click on for all fluorinated pesticides)
TERATOGENICITY RAT
**021 054905, "A Teratology Study of Fluometuron Technical in
the Albino Rat." (Ciba-Geigy, 6-19-86) Fluometuron technical,
batch FL-821838, was administered to mated Crl. COBS CD (SD) (BR)
rats at 0 (3% cornstarch, 0.5% Tween 80), 10, 100, or 1000 mg/kg/day
on days 6 to 15 of gestation, with 27 animals per group. Decreased
food consumption, weight gain, (transient at 100 mg/kg/day) and
darkened spleen were observed at 100 and 1000 mg/kg/day. Lethargy,
ataxia, pale eyes and extremities,
encrustments around eyes/nose/mouth, salivation, blood on vulva,
enlarged spleen and darkened kidneys and liver were observed at
1000 mg/kg/day. Maternal NOEL = 10 mg/kg/day. Delayed renal development
was observed at 100 mg/kg/day. At 1000 mg/kg/day reduced litter
size, fetal weight, and increased incidence
of centrum/vertebra not ossified were observed. Developmental
NOEL = 10 mg/kg/day. Originally reviewed as unacceptable
(M. Silva, 12/15/88), upon receipt of the requested information
regarding analysis of fluometuron in diet, the study was re-reviewed
and found acceptable. M. Silva, 7/25/89.
Ref: Summary of Toxicology Data. California
Department of Food and Agriculture, Medical Toxicology Branch.
Revised October 29, 1989. http://www.fluoridealert.org/pesticides/Fluometuron.CA.EPA.Tox.data.pdf
Blood
(click
on for all fluorinated pesticides)
E. Subchronic Studies. Subchronic feeding studies were conducted
to determine the two concentrations (referred to in this report
as "low" and "high" doses) to be used in the
chronic studies. Fluometuron was administered in the diet for
90 days at doses of 0, 250, 500, 1,000, 2,000, 4,000, 8,000, or
16,000 ppm to groups consisting of 10 males and 10 females of
each species (Tables 1 and 2) (page 5) ... A second 90-day subchronic
study, described in Table 3, was undertaken to investigate in-depth
the effects of feed containing 0 to 4,000 ppm fluometuron on the
spleens of rats.... Gross lesions observed at necropsy included
varying degrees of splenomegaly in all dosed groups. This change
was dose related with the spleens being larger, heavier, darker,
and firmer than the control spleens. In male rats, an increase
in the mean weights of spleens occurred at 1,000 ppm, and the
mean spleen weight at 4,000 ppm was twice that of the control.
In female rats, the mean weight of spleens in the group receiving
250 ppm was greater than that of the control, and those of the
groups receiving 2,000 ppm or 4,000 ppm were respectively twice
and almost three times that of the control.
A dose-related increased incidence of red blood cells with polychromasia
and anisocytosis was observed for both male and female rats. Microscopically,
the pathologic changes were congestion of the red pulp with corresponding
decrease of white pulp. (page 8)
Reference: 1980. Bioassay of fluometuron
for possible carcinogenicty. NCI-CG-TR-195. NTPO-80-11. National
Cancer Institute. Carcinogenesis. Technical Report Series No.
195. NTP No. 80-11.
http://www.fluorideaction.org/pesticides/fluometuron.ntp.1980.pdf
-- Organ Toxicity.
Toxic injury to the liver, kidneys, gut
and brain is induced when lethal doses of fluometuron are administered
experimentally (10). An increase in spleen weight and in the incidence
of abnormalities in red-blood cells,
and decreased weight gain in females were
observed in a 90-day study of rats (18).
-- Carcinogenic Effects. EPA has determined that there is not
enough evidence that fluometuron causes cancer in animals to justify
its classification as a carcinogen. Fluometuron is not classified
as a carcinogen by the EPA (20). An increased incidence of
liver-cell tumors in male mice
was noted in a study of rats and mice. In the same study, no carcinogenic
effects were observed in female mice or in rats of either sex
(18). Mice that were given oral doses of 87 mg/kg for two years
had evidence of liver tumors and
leukemia, a condition characterized by uncontrolled growth in
the number of white blood cells in the blood stream (7).
-- ACUTE TOXICITY. ... It may be fatal if inhaled, swallowed,
or absorbed through skin, as it is irritating to the mucous membrane
lining the skin, gastrointestinal tract, and respiratory system
(2). While there have been no reports of cases of fluometuron
poisoning in humans, this herbicide is considered a mild
inhibitor of cholinesterase. Cholinesterase is an essential
enzyme of the nervous system. Cholinesterase inhibition was observed
in guinea pigs exposed by inhalation to 588 mg/m3 for 2 hours
(18). Fluometuron caused an increased white
blood cell count in agricultural workers (3).
Ref: Flumeturon. EXTOXNET. Pesticide Information
Profile. March 1994.
http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/fluometuron-ext.html
Human
Toxicity Excerpts:
... Mild cholinesterase inhibitor & /causes/ ... an
increased leukocyte count in circulating blood in exposed agricultural
workers. [Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical
Toxicology of Commercial Products. 5th ed. Baltimore: Williams
and Wilkins, 1984.,p. II-331]
Ref: Hazardous Substance Data Bank for Fluometuron.
Available at Toxnet
-- PubMed abstract:
Twelve of fifteen 6-9-mo-old clinically healthy Desert sheep were
given single or repeated daily doses of 25
to 4000 mg cotoran/kg by drench. Cotoran poisoning was characterized
by grinding of the teeth, ruminal tympany, mydriasis, dyspnea,
staggering, paresis of the hind and forelimbs, and recumbency.
Lesions were widespread congestion and hemorrhage, hepatic fatty
change, catarrhal enteritis and degeneration of the epithelial
cells of the renal proximal convoluted tubules. These
were accompanied by significant increases in the activities of
GOT, LDH and GGT and decreases in serum total protein and calcium.
Ref: Vet Hum Toxicol 1995 Jun;37(3):214-6.
Toxicity
of cotoran (fluometuron) in Desert sheep; by Mohamed OS, Ahmed
KE, Adam SE, Idris OF.
Body
Weight Decrease (click
on for all fluorinated pesticides)
--
Organ Toxicity. Toxic injury to the liver,
kidneys, gut and brain is induced when lethal doses of fluometuron
are administered experimentally (10). An increase in spleen weight
and in the incidence of abnormalities in red-blood cells, and
decreased
weight gain in females
were observed in a 90-day study of rats (18).
-- Teratogenic Effects. Pregnant rabbits were given doses of 50,
500 or 1,000 mg/kg/day by gavage during days 6 through 19 of gestation.
An increase in the number of resorbed fetuses was found at all
treatment doses. Reduction in maternal body
weight and food consumption
occurred at doses of 500 and 1,000 mg/kg/day (20).
-- CHRONIC TOXICITY. Rats were fed 7.5, 75, or 750 mg/kg/day for
90 days. At the 750 mg/kg dose, decreased
body weight and congestion in the
spleen, adrenals, liver, and kidneys were evident. The NOAEL for
this study was 7.5 mg/kg/day (100 ppm). When doses of 1.5, 15
or 150 mg/kg/day were fed to puppies for 90 days, congestion of
the liver, kidneys and spleen occurred at the 150 mg/kg
dose. No effects were seen at 15 mg/kg/day (400 ppm) (20).
Ref: Flumeturon. EXTOXNET. Pesticide Information
Profile. March 1994.
http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/fluometuron-ext.html
Chronic Study ** 025
068693, "Fluometuron Technical: 1-Year Oral Adminstration to Dogs
(Min 832047)", (CIBA-Geigy Corporation Pharmaceuticals Div., Laboratory
Study No.832047, May 25, 1988). Fluometuron, purity 95.8%, administered
in the feed at concentrations of 0, 20, 400 or 7000 ppm to Beagle
dogs, 5/sex/group for 52 weeks. Three extra/sex dogs in the control
and the high dose groups were continued an additional 4 weeks
without further treatment and served as the recovery group. No
adverse effect. NOEL = 400 ppm (Increased incidence of emesis;
stool irregularities - diarrhea, mucous, soft and bloody stools;
reduced body weight (14-19% less than control
at week 52), body weight gain and feed consumption;
reduced erythrocytic parameters and indices - HGB, RBC,
HCT, MCV and MCHC; and increases in percent Heinz bodies, Howell-Jolly
bodies and reticulocytes; reduced biochemical parameters - SGPT,
glucose and increased cholesterol, bilirubin, potassium and inorganic
phosphorous levels). Reduced HGB, increased platelet count and
WBC for the recovery group. ACCEPTABLE. (JSK & M. Silva, 8/30/89).
Ref: Summary of Toxicology Data. California
Department of Food and Agriculture, Medical Toxicology Branch.
Revised October 29, 1989.
http://www.fluoridealert.org/pesticides/Fluometuron.CA.EPA.Tox.data.pdf
Bone
(click
on for all fluorinated pesticides)
Developmental Toxicity, Rat (83-3a) MRID: 00163710,42397601 CITATION:
T. Arthur. 1986. A teratoiogy study of fluometuron technical in
the albino rat. Study Number MI# 832125; Tox and Path. Report
No. 199-84. Reproductive and Genetic Toxicology Subdivision Ciba
Geigy Corporation, Summit, NJ 07901. In a developmental study
(MRID 00163710,32397601) fluometuron (95.2% ai, batch # FL 821838)
was administered to 27 female Cr1:COB CD BR rats/dose by gavage
at dose levels of 0, 10, 100, or 1000 mg/kg/day (dosage volume
10 ml/kg/day was adjusted daily for each rats body weight) from
gestation days 6 through 15, inclusive. A dose-related decrease
in fetal weights were observed only in the high-dose group (58%
M, 57% F). No gross observable malformations were observed in
any of the 857 fetuses examined. The fetal M/F sex ratios were
comparable among the groups. A significant
increase in the incidence of shortened or absent renal papillae
was observed in the mid-dose (shortened: 9/82 control, 23/83 high;
absent: 3/82 control, 2/83 high) compared to control.
It should be noted that a statistical analysis was not performed
on the high-dose-due to growth retardations, and reduced fetal
weights. Significant fetal skeletal
variations were observed only in the high-dose group and included
incomplete ossification and widened sutures of the skull, wavy
ribs, and bipartite and fused sternebrae, unossified teeth, vertebral
centra, ischium/Os pubis, metatarsal and the distal phalanges
of the fore-and hind-paws. (pages 51-52).
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
TERATOGENICITY RAT
**021 054905, "A Teratology Study of Fluometuron Technical in
the Albino Rat." (Ciba-Geigy, 6-19-86) Fluometuron technical,
batch FL-821838, was administered to mated Crl. COBS CD (SD) (BR)
rats at 0 (3% cornstarch, 0.5% Tween 80), 10, 100, or 1000 mg/kg/day
on days 6 to 15 of gestation, with 27 animals per group. Decreased
food consumption, weight gain, (transient at 100 mg/kg/day) and
darkened spleen were observed at 100 and 1000 mg/kg/day. Lethargy,
ataxia, pale eyes and extremities, encrustments around eyes/nose/mouth,
salivation, blood on vulva, enlarged spleen and darkened kidneys
and liver were observed at 1000 mg/kg/day. Maternal NOEL = 10
mg/kg/day. Delayed renal development
was observed at 100 mg/kg/day. At 1000 mg/kg/day reduced litter
size, fetal weight, and increased incidence
of centrum/vertebra not ossified were observed. Developmental
NOEL = 10 mg/kg/day. Originally reviewed as unacceptable (M. Silva,
12/15/88), upon receipt of the requested information regarding
analysis of fluometuron in diet, the study was re-reviewed and
found acceptable. M. Silva, 7/25/89.
Ref: Summary of Toxicology Data.
California Department of Food and Agriculture, Medical Toxicology
Branch. Revised October 29, 1989. http://www.fluoridealert.org/pesticides/Fluometuron.CA.EPA.Tox.data.pdf
Brain
(click on for all fluorinated pesticides)
-- Organ Toxicity.
Toxic injury to the liver,
kidneys, gut and brain is induced
when lethal doses of fluometuron are administered experimentally
(TOXNET. 1986. National library of medicine's toxicology data
network. Hazardous Substances Databank. Public Health Service.
National Institute of Health, U. S. Department of Health and Human
Services. Bethesda, MD: NLM.).
Cancer.
Possible Human Carcinogen: LUNG, LYMPHOCYTIC LYMPHOMAS
(click
on for all fluorinated pesticides)
Group
C -- Possible Human Carcinogen.
Statistically significant increases in combined adenomas/carcinomas
of the lung (M); Malignant lymphocytic
lymphomas (F); CD-1 mice.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Group C--Possible
Human Carcinogen. Reviewed 8/ 28/ 96.
Ref:
List of Chemicals Evaluated for Carcinogenic Potential. Science
Information Management Branch, Health Effects Division, Office
of Pesticide Programs, U. S. Environmental Protection Agency.
March 15, 2002.
http://www.biomuncie.org/chemicals_evaluated_for_carcinog.htm
• The
significant cancer risk contributors have been identified as drinking
water (direct and indirect, all sources), and
several rotational crops with wheat (flour), soybean (oil), and
rice (white) having the highest contributions. (page 7)
• A re-examination and statistical analysis of the rates
of all lymphocytic lymphomas in female mice and all alveolarhronchiolar
hyperplasia, adenomas, and carcinomas in male mice from the carcinogenicity
study in mice (0, 10,500, or 2000 ppm [83-2b]) indicated
that male mice had a significant increasing trend in alveolar/bronchiolar
adenomas andor carcinomas combined at p < 0.05. There
were no significant differences in the pair-wise comparisons of
the dosed groups with the controls. The male alveolarhonchiolar
tumor rates were 15/56, 16/59, 19/56, and 24/58, respectively
(L. Taylor Memorandum 9/13/95, TXR 012742). Female
mice had a significant increasing trend and a significant difference
in pair-wise comparison of the high-dose group (2000 ppm) with
the controls for lymphocytic lymphomas at p < 0.05.
The lymphatic tissue tumor rates were 4/5 1, 10124, 6/17, and
12/53, respectively (L. Taylor Memorandum 9/13/95, TXR 012742).(page
9)
• D. Classification of Carcinogenic Potential. The
Health Effects Division Carcinogenicity Peer Review Committee
(CPRC) met on Oct. 11, 1995 to discuss and evaluate the weight-of-the-evidence
on fluometuron with particular reference to its carcinogenic potential.
The consensus of the CPRC was that fluometuron should be classified
as Group C -possible human carcinogen and
for the purpose of risk characterization, both a low dose extrapolation
model (Q,’) applied to the animal data (lung tumors in male
mice) and the Reference Dose (RfD) approach should be used.
This was based on statistically significant increases in combined
adenomadcarcinomas of the lungs in male mice and malignant lymphocytic
lymphomas in female mice, at a dose which was less than
adequate for fully assessing the carcinogenic potential of fluometuron
( L. Taylor Memorandum 8/28/96, TXR 012049).
A quantitative risk assessment-Q,* was performed for fluometuron
(B. Fisher Memorandum 12/24/96, TXR 012809). The unit risk, QI*(mg/kg/day)-’of
fluometuron, based upon male mouse combined lung (adenomas and/or
carcinomas) tumor rates is 1.80 x lo-*in human equivalents (converted
from animals to humans by use of the 314‘s scaling factor-1994,
Tox Risk, 3.5-K. Crump) (Ref. 1).
The dose levels used from this 2-year study were 0, 10,500, and
2000 ppm of fluometuron. The corresponding tumor rates (from re-read
slide data, 7/92) for the male mice were 15/56, 16/59, 19/56,
and 24/58 respectively.
The
estimate of unit risk, Q1*was based upon lung (adenoma and/or
carcinoma) tumor rates in male mice. Since male mice had
statistically significant increases in mortality with dose increments
of fluometuron, the estimate of the unit risk, Q1*,was
obtained by the application of the time-to-tumor Weibull model
(Tox-Risk program, version 3.5-K. Cnunp). The resulting estimate
of unit risk, Q1*,was converted to human equivalents by the use
of weights of 0.03 kg for the mice and 70 kg for the humans and
the 3/4’s scaling factor for interspecies extrapolation.
It is to be noted that Q,* (mg/kg/day)-’ is an estimate
of the upper bound on risk and that (as stated in the EPA Risk
Assessment Guidelines) “the true value of the risk is unknown,
and may be as low as zero. (page 11-12).
(1). See memo-Deriving Q,*s Using
the Unified Interspecies Scaling Factor, P.A. Fenner-Crisp. Director-HED,
7/1/94.
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
Twelve
of the 26 most widely used pesticides in the U.S. are classified
as possible or probable carcinogens by the EPA based on studies
of laboratory animals, with an annual use that totals 380 million
pounds. (atrazine
(C=possible), metolachlor (C), metarn sodium (B2=probable), dichloropropene
(B2), cyanazine (C), pendimethalin (C), trifluralin (C), acetochlor
(B2), chlorothalonil (likely), mancozeb (B2), fluometuron
(C), and parathion (C). Four frequently used pesticides have been
associated with increased risk of cancer for exposed humans in
epidemiological studies. 190 million pounds of these four pesticides
are used annually in the U.S., including 120 million household
applications every year. (atrazine, 2,4 D, glyphosate, diazinon).
(Journal of Pesticide Reform, Summer 1999, at 5). Last
year, the EPA moved under the Food Quality Protection Act to phase
out residential uses of diazinon, but agricultural uses remain
legal.
Ref: Testimony of the Maine Organic Farmers
and Gardeners Association In support of L.D. 1540. March 29, 2001.
http://www.mofga.org/ge_pesticide_test.html
1980 Abstract: ... A bioassay of the phenylurea herbicide fluometuron
for possible carcinogenicity was conducted by administering the
test chemical in feed to F344 rats and B6C3F1 mice. Groups of
50 rats of each sex were fed diets containing 125 or 250 ppm of
fluometuron for 103 weeks, and groups of 50 mice of each sex were
fed diets containing 500 or 1,000 ppm of fluometuron for 103 weeks.
Matched controls consisted of groups of 50 untreated rats and
25 untreated mice of each sex. All surviving animals were killed
at 103 to 105 weeks. Splenomegaly observed
in rats in the subchronic studies influenced selection of the
doses for the chronic study; however, no splenic effects
were observed in the chronic study. Mean body weights of the dosed
groups of male and female rats and mice were essentially the same
as those of the corresponding control groups. Survival of dosed
groups of rats and mice was similar to that of the corresponding
control groups. Similarities between mean body weights and survival
between dosed and control animals in thechronic study suggest
that these animals could have tolerated higher doses. The
only possible carcinogenic effects from compound administration
were in male mice. Incidences of hepatocellular carcinomas or
adenomas in male mice were dose related, and the incidence in
the high-dose group was marginally higher than that in the corresponding
matched controls or pooled controls from concurrent studies.
Under the conditions of this bioassay, fluometuron was not carcinogenic
for F344 rats or for female B6C3F1 mice. Equivocal
results were obtained for male B6C3F1 mice which may have had
an increased incidence of hepatocellular tumors. Because
of the equivocal findings and because both rats and mice may have
been able to tolerate higher doses, it is concluded that additional
testing of fluometuron for carcinogenicity is warranted. Levels
of Evidence of Carcinogenicity: Male Rats: Negative Female Rats:
Negative Male Mice: Equivocal Female Mice: Negative Synonym: 1,1-dimethyl-3-(a,a,a-trifluoro-m-tolyl)
urea
Ref: Bioassay of Fluometuron for Possible
Carcinogenicity (CAS No. 2164-17-2). National Toxicology Program.
Natl Toxicol Program Tech Rep Ser. 1980 Aug;195:1-99.
[Abstract from Toxline at Toxnet]
CNS
(click
on for all fluorinated pesticides)
-- ACUTE TOXICITY.
... It may be fatal if inhaled, swallowed, or absorbed through
skin, as it is irritating to the mucous membrane lining the skin,
gastrointestinal tract, and respiratory system (2). While there
have been no reports of cases of fluometuron poisoning in humans,
this herbicide is considered a mild inhibitor
of cholinesterase. Cholinesterase
is an essential enzyme of the nervous system.
Cholinesterase inhibition was observed in guinea pigs exposed
by inhalation to 588 mg/m3 for 2 hours (18).
Fluometuron caused an increased white blood cell count in agricultural
workers (3).
Ref: Flumeturon. EXTOXNET. Pesticide Information
Profile. March 1994.
http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/fluometuron-ext.html
Endocrine:
Adrenal (click
on for all fluorinated pesticides)
-- CHRONIC TOXICITY.
Rats were fed 7.5, 75, or 750 mg/kg/day
for 90 days. At the 750 mg/kg dose, decreased body weight and
congestion in the spleen,
adrenals, liver, and kidneys
were evident. The NOAEL for this study was 7.5 mg/kg/day (100
ppm). When doses of 1.5, 15 or 150 mg/kg/day were fed to puppies
for 90 days, congestion of the liver, kidneys and spleen occurred
at the 150 mg/kg dose. No effects were seen at 15 mg/kg/day (400
ppm) (20).
Ref: Flumeturon. EXTOXNET. Pesticide Information
Profile. March 1994.
http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/fluometuron-ext.html
Endocrine:
Pituitary (click
on for all fluorinated pesticides)
A histological re-examination and statistical analysis of pituitary
adenomas in Fischer 344 rats from a combined chronic toxicity/oncogenicity
study (0, 10, 300, or 1000 ppm [83-51) indicated no significant
increasing trends in male rats, but a significant
difference was observed in a pairwise comparison of the 10 and
1000 ppm dose groups with the controls for pituitary adenomas
was significant at p < 0.05, and a significant difference observed
in the pair-wise comparison of the 300 ppm dose group with the
controls for pituitary adenomas at p < 0.01. The pituitary
adenoma tumor rates in male rats were 3/47,9/51, 1 1/51, and 9/58,
respectively (L. Taylor Memorandum 9/13/95. TXR 012742). There
were no compound related tumors observed in female rats. Two other
studies submitted by the National Cancer Institute (NCI) for both
mice and rats were either unremarkable or the results equivocal.
However, genotoxicity studies with fluometuron were negative.
(page 9)
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
Endocrine:
Testicular
(click on for all fluorinated pesticides)
-- Mutagenic Effects.
In two separate assays, one on yeast and the other on bacterial
cell cultures, fluometuron failed to cause mutations. Fluometuron
interfered with DNA synthesis in the testes of mice given
a single oral dose of 2,000 mg/kg (20).
Ref: Flumeturon. EXTOXNET. Pesticide Information
Profile. March 1994.
http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/fluometuron-ext.html
Eye
(click on for all fluorinated pesticides)
Primary
eye irritation: Induces corneal opacity,
Toxicity Category I. Fluometuron
(Cotoran, Lanex) Herbicide Profile 12/85.
Ref: Chemical Fact Sheet for Fluometuron.
Fact Sheet Number 88. December 1985.
http://pmep.cce.cornell.edu/profiles/herb-growthreg/fatty-alcohol-monuron/fluometuron/herb-prof-fluometuron.html
• Fluometuron is of low to moderate toxicity with toxicity
categories of III and IV, except for dermal and eye
irritation (II). (page 2)
• Primary eye irritation studies indicate fluometuron produces
corneal opacity, iris irritation, redness,
chemosis, and discharge, all of which cleared from day
4 to day 10 (Tox Category II) (page
8)
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
TERATOGENICITY RAT
**021 054905, "A Teratology Study of Fluometuron Technical in
the Albino Rat." (Ciba-Geigy, 6-19-86) Fluometuron technical,
batch FL-821838, was administered to mated Crl. COBS CD (SD) (BR)
rats at 0 (3% cornstarch, 0.5% Tween 80), 10, 100, or 1000 mg/kg/day
on days 6 to 15 of gestation, with 27 animals per group. Decreased
food consumption, weight gain, (transient at 100 mg/kg/day) and
darkened spleen were observed at 100 and 1000 mg/kg/day. Lethargy,
ataxia, pale
eyes and extremities, encrustments around eyes/nose/mouth,
salivation, blood on vulva, enlarged spleen and darkened kidneys
and liver were observed at 1000 mg/kg/day. Maternal NOEL = 10
mg/kg/day. Delayed renal development was observed at 100 mg/kg/day.
At 1000 mg/kg/day reduced litter size, fetal weight, and
increased incidence of centrum/vertebra not ossified were observed.
Developmental NOEL = 10 mg/kg/day. Originally reviewed
as unacceptable (M. Silva, 12/15/88), upon receipt of the requested
information regarding analysis of fluometuron in diet, the study
was re-reviewed and found acceptable. M. Silva, 7/25/89.
Ref: Summary of Toxicology Data. California
Department of Food and Agriculture, Medical Toxicology Branch.
Revised October 29, 1989. http://www.fluoridealert.org/pesticides/Fluometuron.CA.EPA.Tox.data.pdf
Fluometuron is a mild
skin and eye irritant. It has caused skin sensitization in guinea
pigs and in humans. It affects the cornea
of the eye in such a way that light cannot pass through
it. This condition is referred to as corneal opacity. Skin or
eye contact with it may cause burning... Prolonged or repeated
exposure to fluometuron may cause conjunctivitis.
Ref: Pesticide Informationnn Profile. Fluometuron.
March 1994. EXTOXNET.
http://www.fluoridealert.org/pesticides/Fluometuron.Extoxnet.1994.htm
Kidney
(click
on for all fluorinated pesticides)
• The spleen, kidney,
and liver appear to be the organs consistently
affected following exposure to moderate doses of fluometuron
in rats and dogs in subchronic, chronic, developmental, and reproductive
studies. (page 8)
• 3. Dermal Exposure. Short-Term (1-30 days) However, the
developmental rat study (MRID 00163710) can be used for the short-term
dermal exposure based on the developmental NOAEL of 10 mg/kg/day
and LOAEL of 100 mg/kg/day, based on delayed
urinary system development (shortened renal papillae).
This effect can be assumed to have occurred during the dosing
period but delayed until later in development. This provides a
conservative endpoint for short-term exposure.
• 4. Inhalation Exposure (All Durations). A. Short -Term
(1-30 Days) The developmental rat study (MRID 00 163710) encompasses
the appropriate exposure duration (gestation days 6-15) with developmental
NOAEL of 10 mg/kg/day and LOAEL of 100 mg/kg/day, based
on delayed urinary system development
(shortened renal papillae). This study is also supported
by the developmental rabbit study (MRID 00147554) in which the
maternal NOAEL was also 10 mg/kg/day and LOAEL 100 mg/kg/day,
based on increased incidence of stool variations, increased incidence
of abortions, reduced food consumption and decreased maternal
body weight gain.
• Developmental Toxicity, Rat (83-3a) MRID: 00163710,42397601
CITATION: T. Arthur. 1986. A teratoiogy study of fluometuron technical
in the albino rat. Study Number MI# 832125; Tox and Path. Report
No. 199-84. Reproductive and Genetic Toxicology Subdivision Ciba
Geigy Corporation, Summit, NJ 07901. In a developmental study
(MRID 00163710,32397601) fluometuron (95.2% ai, batch # FL 821838)
was administered to 27 female Cr1:COB CD BR rats/dose by gavage
at dose levels of 0, 10, 100, or 1000 mg/kg/day (dosage volume
10 ml/kg/day was adjusted daily for each rats body weight) from
gestation days 6 through 15, inclusive. A dose-related decrease
in fetal weights were observed only in the high-dose group (58%
M, 57% F). No gross observable malformations were observed in
any of the 857 fetuses examined. The fetal M/F sex ratios were
comparable among the groups. A significant
increase in the incidence of shortened or absent renal papillae
was observed in the mid-dose (shortened: 9/82 control, 23/83 high;
absent: 3/82 control, 2/83 high) compared to control.
It should be noted that a statistical analysis was not performed
on the high-dose-due to growth retardations, and reduced fetal
weights. Significant fetal skeletal
variations were observed only in the high-dose group and included
incomplete ossification and widened sutures of the skull, wavy
ribs, and bipartite and fused sternebrae, unossified teeth, vertebral
centra, ischium/Os pubis, metatarsal and the distal phalanges
of the fore-and hind-paws. (pages 51-52).
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
TERATOGENICITY RAT
**021 054905, "A Teratology Study of Fluometuron Technical in
the Albino Rat." (Ciba-Geigy, 6-19-86) Fluometuron technical,
batch FL-821838, was administered to mated Crl. COBS CD (SD) (BR)
rats at 0 (3% cornstarch, 0.5% Tween 80), 10, 100, or 1000 mg/kg/day
on days 6 to 15 of gestation, with 27 animals per group. Decreased
food consumption, weight gain, (transient at 100 mg/kg/day) and
darkened spleen were observed at 100 and 1000 mg/kg/day. Lethargy,
ataxia, pale eyes and extremities,
encrustments around eyes/nose/mouth, salivation, blood on vulva,
enlarged spleen and darkened kidneys and liver were observed at
1000 mg/kg/day. Maternal NOEL = 10 mg/kg/day. Delayed
renal development was observed at 100 mg/kg/day. At 1000
mg/kg/day reduced litter size, fetal weight, and
increased incidence of centrum/vertebra not ossified were observed.
Developmental NOEL = 10 mg/kg/day. Originally reviewed
as unacceptable (M. Silva, 12/15/88), upon receipt of the requested
information regarding analysis of fluometuron in diet, the study
was re-reviewed and found acceptable. M. Silva, 7/25/89.
Ref: Summary of Toxicology Data. California
Department of Food and Agriculture, Medical Toxicology Branch.
Revised October 29, 1989. http://www.fluoridealert.org/pesticides/Fluometuron.CA.EPA.Tox.data.pdf
-- Organ Toxicity.
Toxic injury to the liver,
kidneys, gut and brain
is induced when lethal doses of fluometuron are administered experimentally
(10). An increase in spleen weight and in the incidence of abnormalities
in red-blood cells, and decreased weight gain in females were
observed in a 90-day study of rats (18).
-- CHRONIC TOXICITY. Rats were fed 7.5, 75, or 750 mg/kg/day for
90 days. At the 750 mg/kg dose, decreased
body weight and congestion in the spleen, adrenals, liver, and
kidneys were
evident. The NOAEL for this study was 7.5 mg/kg/day (100 ppm).
When doses of 1.5, 15 or 150 mg/kg/day were fed to puppies for
90 days, congestion of the liver, kidneys
and spleen
occurred at the 150 mg/kg dose. No effects were seen at 15 mg/kg/day
(400 ppm) (20).
Ref: Flumeturon. EXTOXNET. Pesticide Information
Profile. March 1994.
http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/fluometuron-ext.html
-- PubMed abstract:
Twelve of fifteen 6-9-mo-old clinically healthy Desert sheep were
given single or repeated daily doses of 25 to 4000 mg cotoran/kg
by drench. Cotoran poisoning was characterized by
grinding of the teeth, ruminal tympany, mydriasis, dyspnea, staggering,
paresis of the hind and forelimbs, and recumbency. Lesions were
widespread congestion and hemorrhage, hepatic fatty change, catarrhal
enteritis and degeneration of the
epithelial cells of the renal proximal convoluted tubules. These
were accompanied by significant increases in the activities of
GOT, LDH and GGT and decreases in serum total protein and calcium.
Ref: Vet Hum Toxicol 1995 Jun;37(3):214-6.
Toxicity
of cotoran (fluometuron) in Desert sheep; by Mohamed OS, Ahmed
KE, Adam SE, Idris OF.
Leukemia
(click on for all fluorinated
pesticides)
EPA
has determined that there is not enough evidence that fluometuron
causes cancer in animals to justify its classification as a carcinogen.
Fluometuron is not classified as a carcinogen by the EPA (20).
An increased incidence of liver-cell tumors in male mice was noted
in a study of rats and mice. In the same study, no carcinogenic
effects were observed in female mice or in rats of either sex
(18). Mice that were given oral doses of 87 mg/kg for two years
had evidence of liver tumors and leukemia,
a condition characterized by uncontrolled growth in the number
of white blood cells in the blood stream (National Institute
for Occupational Safety and Health (NIOSH). 1986. Registry of
toxic effects of chemical substances (RTECS). Cincinnati, OH:
NIOSH.)
Ref:
1994 Pesticide Information Profile. EXTOXNET.
http://www.fluoridealert.org/pesticides/fluometuron.extoxnet.1994.htm
Liver
(click on for all fluorinated pesticides)
The spleen, kidney, and
liver appear to be the organs consistently
affected following exposure to moderate doses of fluometuron
in rats and dogs in subchronic, chronic, developmental, and reproductive
studies. (page 8)
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
Groups of 50 /B6C3F1/
mice of each sex were fed diets containing 500 or 1,000 ppm of
fluometuron for 103 weeks. Matched controls consisted of groups
of 25 untreated mice of each sex. All surviving animals were killed
at 103 to 105 weeks. Mean body weights of the dosed groups of
male and female mice were essentially the same as those of the
corresponding control groups. Survival of dosed groups of mice
were similar to that of the corresponding control groups. Similarities
between mean body weights and survival between dosed and control
animals in the chronic study suggest that these animals could
have tolerated higher doses. The only possible carcinogenic effects
from compound administration were in male
mice. Incidences of hepatocellular
carcinomas or adenomas in male mice were dose
related, and the incidence in the
high-dose group was marginally higher than that in the corresponding
matched controls or pooled controls from concurrent studies.
Under the conditions of this bioassay, fluometuron was
not carcinogenic for F344 rats or for female B6C3F1 mice.
Equivocal results were obtained for male B6C3F1 mice which may
have had an increased incidence of hepatocellular tumors.
Because of the equivocal findings and because both rats and mice
may have been able to tolerate higher doses,
it is concluded that additional testing of fluometuron for carcinogenicity
is warranted. (Summary page v)
Reference: 1980. Bioassay of fluometuron
for possible carcinogenicty. NCI-CG-TR-195. NTPO-80-11. National
Cancer Institute. Carcinogenesis. Technical Report Series No.
195. NTP No. 80-11.
http://www.fluorideaction.org/pesticides/fluometuron.ntp.1980.pdf
PubMed abstract: The
experiments on the investigation of pesticide fluometuron (cotoran)
influence on nuclease sensitivity and template activity of rat
liver chromatin were carried out. Cotoran was found to
bind specifically with non-histone proteins of chromatin. It was
shown that this pesticide considerably decreases template activity
of chromatin and its sensitivity to the action of nucleases. It
suggests, that certain conformation changes occur in chromatin
upon the action of cotoran.
Ref: Biull Eksp Biol Med 1992 Mar;113(3):261-3.
[Effects of pesticide fluometuron (cotoran) on template synthesis
of RNA] [Article in Russian] Khamidov DKh, Mirakhmedov AK, Sagatova
GA, Azimova ShS. PMID: 1384777 [PubMed - indexed for MEDLINE]
-- Organ Toxicity.
Toxic injury to the liver, kidneys,
gut and brain is induced when
lethal doses of fluometuron are administered experimentally (10).
An increase in spleen weight and in the incidence of abnormalities
in red-blood cells, and decreased weight gain in females were
observed in a 90-day study of rats (18).
-- Carcinogenic Effects. EPA has determined that there is not
enough evidence that fluometuron causes cancer in animals to justify
its classification as a carcinogen. Fluometuron is not classified
as a carcinogen by the EPA (20). An increased incidence of
liver-cell tumors in male mice was noted in a study of
rats and mice. In the same study, no carcinogenic effects were
observed in female mice or in rats of either sex (18). Mice that
were given oral doses of 87 mg/kg for two years had evidence of
liver tumors and leukemia,
a condition characterized by uncontrolled growth in the number
of white blood cells in the blood stream (7).
-- CHRONIC TOXICITY. Rats were fed 7.5, 75, or 750 mg/kg/day for
90 days. At the 750 mg/kg dose, decreased
body weight and congestion in the
spleen, adrenals, liver,
and kidneys
were evident.
The NOAEL for this study was 7.5 mg/kg/day (100 ppm). When doses
of 1.5, 15 or 150 mg/kg/day were fed to puppies for 90 days, congestion
of the liver, kidneys
and spleen
occurred at the
150 mg/kg dose. No effects were seen at 15 mg/kg/day (400 ppm)
(20).
Ref: Fluometuron. EXTOXNET. Pesticide Information
Profile. March 1994. http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/fluometuron-ext.html
-- PubMed abstract:
Twelve of fifteen 6-9-mo-old clinically healthy Desert sheep were
given single or repeated daily doses of 25 to 4000 mg cotoran/kg
by drench. Cotoran poisoning was characterized
by grinding of the teeth, ruminal tympany, mydriasis, dyspnea,
staggering, paresis of the hind and forelimbs, and recumbency.
Lesions were widespread congestion and hemorrhage, hepatic
fatty change, catarrhal enteritis and degeneration
of the epithelial cells of the renal proximal convoluted tubules.
These were accompanied by significant increases in the activities
of GOT, LDH and GGT and decreases in serum total protein and calcium.
Ref: Vet Hum Toxicol 1995 Jun;37(3):214-6.
Toxicity
of cotoran (fluometuron) in Desert sheep; by Mohamed OS, Ahmed
KE, Adam SE, Idris OF.
-- PubMed abstract:
The experiments on the investigation of pesticide cotoran-effect
on RNA synthesis and transport were carried out. Cotoran was shown
to destroy considerably the processes of
RNA biosynthesis in rat liver, that results in the decrease
of RNA transport from nuclei into cytoplasm. By special experiments
it was established that functional activity and the integrity
of nuclear membrane (according to the alteration in the activity
of nuclear membrane enzyme Mg2-dependent ATP-ase) was not destroyed.
Ref: Biull Eksp Biol Med 1992 Jan;113(1):40-2.
[RNA
synthesis and transport in the rat liver under the effects of
pesticide cotoran (fluometuron)]. [Article in Russian]; by
Khamidov DKh, Marakhmedov AK, Sagatova GA, Azimova ShS.
LYMPHOMAS
(click
on for all fluorinated pesticides)
Group
C -- Possible Human Carcinogen.
Statistically significant increases in combined adenomas/carcinomas
of the lung (M); Malignant
lymphocytic lymphomas (F); CD-1 mice.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Lung
(click
on for all fluorinated pesticides)
Group
C -- Possible Human Carcinogen.
Statistically significant increases in combined adenomas/carcinomas
of the lung (M); Malignant lymphocytic
lymphomas (F); CD-1 mice.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
• A re-examination and statistical
analysis of the rates of all lymphocytic lymphomas in female mice
and all alveolarhronchiolar hyperplasia, adenomas, and carcinomas
in male mice from the carcinogenicity study in mice (0, 10,500,
or 2000 ppm [83-2b]) indicated that male
mice had a significant increasing trend in alveolar/bronchiolar
adenomas andor carcinomas combined at p < 0.05. There
were no significant differences in the pair-wise comparisons of
the dosed groups with the controls. The male alveolarhonchiolar
tumor rates were 15/56, 16/59, 19/56, and 24/58, respectively
(L. Taylor Memorandum 9/13/95, TXR 012742). Female mice had a
significant increasing trend and a significant difference in pair-wise
comparison of the high-dose group (2000 ppm) with the controls
for lymphocytic lymphomas at p < 0.05. The lymphatic tissue
tumor rates were 4/5 1, 10124, 6/17, and 12/53, respectively (L.
Taylor Memorandum 9/13/95, TXR 012742).(page 9)
• D. Classification of Carcinogenic Potential. The
Health Effects Division Carcinogenicity Peer Review Committee
(CPRC) met on Oct. 11, 1995 to discuss and evaluate the weight-of-the-evidence
on fluometuron with particular reference to its carcinogenic potential.
The consensus of the CPRC was that fluometuron should be classified
as Group C -possible human carcinogen and
for the purpose of risk characterization, both a low dose extrapolation
model (Q,’) applied to the animal data (lung tumors in male
mice) and the Reference Dose (RfD) approach should be used.
This was based on statistically significant increases in combined
adenomadcarcinomas of the lungs in male mice and
malignant lymphocytic lymphomas in female mice,
at a dose which was less than adequate for fully assessing the
carcinogenic potential of fluometuron ( L. Taylor Memorandum 8/28/96,
TXR 012049).
A quantitative risk assessment-Q,* was performed for fluometuron
(B. Fisher Memorandum 12/24/96, TXR 012809). The unit risk, QI*(mg/kg/day)-’of
fluometuron, based upon male mouse combined lung (adenomas and/or
carcinomas) tumor rates is 1.80 x lo-*in human equivalents (converted
from animals to humans by use of the 314‘s scaling factor-1994,
Tox Risk, 3.5-K. Crump) (Ref. 1).
The dose levels used from this 2-year study were 0, 10,500, and
2000 ppm of fluometuron. The corresponding tumor rates (from re-read
slide data, 7/92) for the male mice were 15/56, 16/59, 19/56,
and 24/58 respectively.
The
estimate of unit risk, Q1*was based upon lung (adenoma and/or
carcinoma) tumor rates in male mice. Since male mice had
statistically significant increases in mortality with dose increments
of fluometuron, the estimate of the unit risk, Q1*,was
obtained by the application of the time-to-tumor Weibull model
(Tox-Risk program, version 3.5-K. Cnunp). The resulting estimate
of unit risk, Q1*,was converted to human equivalents by the use
of weights of 0.03 kg for the mice and 70 kg for the humans and
the 3/4’s scaling factor for interspecies extrapolation.
It is to be noted that Q,* (mg/kg/day)-’ is an estimate
of the upper bound on risk and that (as stated in the EPA Risk
Assessment Guidelines) “the true value of the risk is unknown,
and may be as low as zero. (page 11-12).
(1). See memo-Deriving Q,*s Using
the Unified Interspecies Scaling Factor, P.A. Fenner-Crisp. Director-HED,
7/1/94.
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
Reproductive/Developmental
(click on for all fluorinated pesticides)
E. Endpoint Selection or Dose/Response
1. Acute Reference Dose For the general population, no study exists
to determine effects from a single dose. Therefore there is no
acute reference dose for the general population. However, for
females age 13+ (women of child bearing years) there is a study
to support this reference dose. In the rat
developmental toxicity study (83-3aYMRID 00163710), dose
levels of 10, 100, and 1000 mg/kg/day were administered via gavage
on days 6-15 of gestation. The NOAEL/LOAEL for developmental toxicity
were 10/100 mg/kg/day, based on delayed
urinary system development (23/83 [28%] vs. 9/82 [11%]
controls). The urinary system of the fetus
forms at specific time points during development and therefore
could be adversely affected from a single exposure during gestation.
Delayed urinary development would not be
recognized until later during growth. Maternal NOAEL/LOAEL
was 10/100 mg/kg/day, based on decreased food consumption (high-dose:
8%-23% from Day 10 to Day 20 of gestation) and an increased
incidence of darkened spleens (mid: 7/27 [26%]; high: 24/27
[89%], controls: 0/27). An uncertainty factor of 100 (1 OX for
interspecies extrapolation and 1OX for intraspecies variability)
is applied.
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
Spleen
(click
on for all fluorinated pesticides)
The spleen, kidney, and liver appear
to be the organs consistently affected
following exposure to moderate doses of fluometuron in rats and
dogs in subchronic, chronic, developmental, and reproductive studies.
(page 8)
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk Assessment
for Phase III of the Reregistration Eligibility Decision. Docket
Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
E. Subchronic Studies. Subchronic feeding studies were conducted
to determine the two concentrations (referred to in this report
as "low" and "high" doses) to be used in the
chronic studies. Fluometuron was administered in the diet for
90 days at doses of 0, 250, 500, 1,000, 2,000, 4,000, 8,000, or
16,000 ppm to groups consisting of 10 males and 10 females of
each species (Tables 1 and 2) (page 5) ... A second 90-day subchronic
study, described in Table 3, was undertaken to investigate in-depth
the effects of feed containing 0 to 4,000 ppm fluometuron on the
spleens of rats.... Gross lesions observed
at necropsy included varying degrees of splenomegaly in all dosed
groups. This change was dose related with the spleens being larger,
heavier, darker, and firmer than the control spleens. In male
rats, an increase in the mean weights of spleens occurred at 1,000
ppm, and the mean spleen weight at 4,000 ppm was twice that of
the control. In female rats, the mean weight of spleens in the
group receiving 250 ppm was greater than that of the control,
and those of the groups receiving 2,000 ppm or 4,000 ppm were
respectively twice and almost three times that of the control.
A dose-related increased incidence of red
blood cells with polychromasia and anisocytosis was observed for
both male and female rats. Microscopically, the pathologic changes
were congestion of the red pulp with corresponding decrease of
white pulp. (page 8)
Reference: 1980. Bioassay of fluometuron
for possible carcinogenicty. NCI-CG-TR-195. NTPO-80-11. National
Cancer Institute. Carcinogenesis. Technical Report Series No.
195. NTP No. 80-11.
http://www.fluorideaction.org/pesticides/fluometuron.ntp.1980.pdf
-- CHRONIC
TOXICITY. Rats were fed 7.5, 75, or 750 mg/kg/day for 90 days.
At the 750 mg/kg dose, decreased body weight
and congestion in the spleen,
adrenals, liver, and kidneys
were evident. The NOAEL for this study was 7.5 mg/kg/day (100
ppm). When doses of 1.5, 15 or 150 mg/kg/day were fed to puppies
for 90 days, congestion of the liver, kidneys and
spleen
occurred at the 150 mg/kg dose. No effects were seen at 15 mg/kg/day
(400 ppm) (20).
Ref: Flumeturon. EXTOXNET. Pesticide
Information Profile. March 1994.
http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/fluometuron-ext.html
| Environmental
(click on for all fluorinated
pesticides)
D.
Environmental Fate and Drinking Water Exposure and Risk
Assessment
Fluometuron and its metabolites
are mobile and persistent in the environment. The primary
route of degradation of fluometuron and its main degradate
CGA-41686 [1-methyl-3-(a,a,a-trifluoro-m-tolyl)urea] is microbial metabolism.
However, since fluometuron and its degradates are not
volatile, and these degradative processes are not rapid
enough, these compounds will be available for leaching
to ground water and runoff to surface water in many use
conditions. Once in ground water or surface water, fluometuron
is expected to persist due to its stability to hydrolysis
and photolysis. (page 39)
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk
Assessment for Phase III of the Reregistration Eligibility
Decision. Docket Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
Fluometuron
is an urea herbicide used for annual grass and annual
broadleaf weed control in cotton. Its use on cotton poses
risks on an acute basis to both endangered and non-endangered
freshwater fish, invertebrates, birds, mammals, and aquatic
plants. The Agency was unable to
assess the potential chronic risk to birds, and freshwater
or estuarine/marine fish and invertebrates due to insufficient
data. (page 1)
Major
Conclusions
Risks to Terrestrial Organisms
• There are acute risks to
avian species that feed on short grass, long grass,
and broadleaf plants/small insects. C Chronic risk to
birds could not be evaluated due to absence
of chronic avian testing.
• There are acute risks to
mammals (15 g and 35 g) that forage on short grass,
long grass, and broadleaf plants/small insects. There
is acute risk to large mammals (1000 g) that feed on short
grass.
• There is chronic risk to
mammals that forage on short grass, long grass,
broadleaf plants/small insects, and fruits/pods/large
insects/seeds.
• Risk to terrestrial plants
on land and in semi-aquatic areas is expected.
Risks to Aquatic Organisms
• The present assessment suggests potential risk
on an acute basis to freshwater fish and invertebrates.
These estimates were based on Mississippi (MS), Texas
(TX) and North Carolina (NC) cotton scenarios (endangered
and non-endangered species’ LOCs exceeded). For
the California cotton scenario, only endangered species’
LOCs are exceeded for freshwater fish and invertebrates.
• Although estuarine/marine invertebrates endangered
species’ LOCs are exceeded, there are currently
no federally listed threatened or endangered estuarine/marine
invertebrates.
• The Agency was unable to assess the potential
chronic risk to freshwater and estuarine/marine fish or
freshwater and estuarine/marine invertebrates due
to data gaps.
• Risk to aquatic plants is expected for both non-endangered
and endangered species.
D.
Key Uncertainties and Information Gaps
The following uncertainties and information gaps were
identified as part of the problem formulation:
• Chronic data for birds
were not submitted by the registrant;
therefore, measurement endpoints could not be estimated.
• Chronic data for freshwater
fish were not submitted by
the registrant; therefore, measurement endpoints
could not be estimated.
• Chronic data for freshwater
invertebrates were not submitted
by the registrant; therefore, measurement endpoints
could not be estimated.
• Chronic data for estuarine/marine
fish were not submitted by
the registrant; therefore, measurement endpoints
could not be estimated .
• Chronic data for estuarine/marine
invertebrates and mollusks were not
submitted by the registrant; therefore, measurement
endpoints could not be estimated.
• Inhalation and dermal pathways for terrestrial
mammals and birds were not evaluated because these routes
of exposure are considered to be negligible compared to
the dietary ingestion pathways. Uncertainties associated
with exposure pathways for terrestrial animals are discussed
in greater detail in Section IV.D.3.
• Risks to semiaquatic wildlife via consumption
of pesticide-contaminated fish were not evaluated. However,
given that bioaccumulation of fluometuron is low, ingestion
of fish by piscivorus wildlife is not likely to be of
concern.
• Risks to top-level carnivores
were not evaluated due to
a lack of data for these receptors. Ingestion of
grass, plants, fruits, insects, and seeds by terrestrial
wildlife was considered; however, consumption of small
mammals and birds by carnivores was not evaluated. In
addition, food chain exposures for aquatic receptors (i.e.,
fish consumption of aquatic invertebrates and/or aquatic
plants) were also not considered.
• Surrogates were used to
predict potential risks for species with no data (i.e.,
reptiles and amphibians). It was assumed that use of surrogate
effects data is sufficiently conservative to apply the
broad range of species within taxonomic groups. If other
species are more or less sensitive to fluometuron than
the surrogates, risks may be under or overestimated, respectively.
The
preliminary risk assessment for endangered species indicates
that fluometuron exceeds the endangered
species LOCs [Level of Concern] for the following combinations
of analyzed uses and species: (page
39)
• Freshwater fish (acute): Cotton (all scenarios
modeled - MS, NC, TX, and CA).
• Freshwater invertebrates (acute): Cotton (all
scenarios modeled - MS, NC, TX, and CA).
• Estuarine/marine invertebrates (acute): Cotton
(scenarios modeled - MS, NC, and TX). CA cotton scenario
does not exceed endangered species’ LOCs.
• Aquatic vascular plants (acute): Cotton (MS, TX,
and NC scenarios).
• Aquatic non-vascular plants (acute): Cotton (MS,
TX, and NC scenarios).
• Birds (acute) : Cotton (short grass, tall grass,
and broadleaf plants/small insects).
• Mammals (acute) : Cotton (short grass, tall grass,
broadleaf plants/small insects) for small (15 g) and medium
(35 g) mammal and cotton (short grass) for large mammals
(1000 g).
• Mammals (chronic) : Cotton ( short grass, tall
grass, broadleaf plants/small insects, and fruits/pods/large
insects/seeds).
• Non-target terrestrial and semi-aquatic plants
(acute): Cotton (dry areas, wetland areas, and drift).
Ref.
February 1, 2005. US EPA: Fluometuron: Revised HED Risk
Assessment for Phase III of the Reregistration Eligibility
Decision. Docket Identification Number: OPP-2004-0372-0008.
http://www.fluorideaction.org/pesticides/fluometuron.opp-2004-0372-0008.pdf
/ACUTE
SYMPTOMS IN MALLARDS AFTER
ORAL ADMIN ARE/ ATAXIA, WING DROP
OR WINGS CROSSED HIGH OVER BACK, TAIL POINTED UPWARD, FLUFFED
FEATHERS, HYPEREXCITABILITY, PHONATION, FALLING. SIGNS APPEARED
15 MIN AFTER-TREATMENT & PERSISTED FOR UP TO ONE WK.
[U.S. Department of the Interior, Fish and Wildlife Service.
Handbook of Toxicity of Pesticides to Wildlife. Resource
Publication 153. Washington, DC: U.S. Government Printing
Office, 1984. 44]
Environmental
Fate: TERRESTRIAL FATE: IT IS OF INTERMEDIATE
PERSISTENCE WITH A HALF-LIFE OF 60-75 DAYS ACCORDING
TO SOIL CONDITIONS. [Worthing, C.R. and S.B. Walker (eds.).
The Pesticide Manual - A World Compendium. 8th ed. Thornton
Heath, UK: The British Crop Protection Council, 1987.
412]
Environmental
Bioconcentration: An estimated BCF of 15 was calculated
for fluometuron(SRC), using a log Kow of 2.42 (1) and
a regression-derived equation(2). According to a classification
scheme(3), this BCF suggests the potential for bioconcentration
in aquatic organisms is low. However,
in a study of unicellular green algae (Chlorella fusca),
a log BCF of 1.96(4) was determined for fluometuron, corresponding
to a BCF of 91.2 and indicating
a moderate potential for bioconcentration in algae.
[(1) Hansch C et al; Exploring QSAR. Hydrophobic, Electronic,
and Steric Constants. ACS Prof Ref Book. Heller SR, consult.
ed., Washington, DC: Amer Chem Soc p. 70 (1995) (2) Meylan
WM et al; Environ Toxicol Chem 18: 664-72 (1999) (3) Franke
C et al; Chemosphere 29: 1501-14 (1994) (4) Manthey M
et al; pp. 453-459 in The Sci of the Total Environ, Supp.
Amsterdam, The Netherlands: Elsevier (1993)]
Environmental
Water Concentrations: GROUNDWATER:
Based on the records maintained in the STORET database
of EPA, fluometuron was not detected in any of the 156
groundwater samples analyzed from 125 locations in the
U.S.(1). Fluometuron was not detected (detection limit
0.5 ug/l) in water from 119 wells, springs and municipal
drinking water supplies sampled throughout Arkansas, during
1985-1987(2). In a study of groundwater sampled in 1992-1998
from 231 wells in 14 counties in
the Arkansas Delta, fluometuron was detected at high and
persistent concentrations in two of the wells,
at 0.4-0.9 ug/l in a well used for a machine shop and
at 19-24 ug/l in a well designated
for domestic use(3). [(1) USEPA; Drinking Water
Health Advisory: Pesticides, Chelsea, MI: Lewis Publishers,
Inc. p. 427-41 (1989) (2) Cavalier TC et al; Ground Water
Monit Rev 9: 159-66 (1989) (3) Nichols T et al; Water
Res Engr 98: 1242-1247 (1998)]
SURFACE
WATER:
Based on the records maintained in the STORET database
of EPA, fluometuron was not detected in 14 surface water
samples from 14 locations in the US(1).
In a study of the Mississippi River and its tributaries
in July/Aug 1991, fluometuron was detected in three tributaries
and at three Mississippi River sites at concns of 9-411
ng/l(2). [(1) USEPA; Drinking Water Health Advisory:
Pesticides. Chelsea, MI: Lewis Publishers, Inc. p. 427-41
(1989) (2) Pereira WE, Hostettler FD; Environ Sci Tech
27: 1542-1552 (1993)]
Ref:
Hazardous Substance Data Bank for Fluometuron. Available
at Toxnet
Abstract:
Existing drinking water wells are widely used for the
collection of ground water samples to evaluate chemical
contamination. A well comparison study was conducted to
compare pesticide and nitrate-N data from specially designed
stainless steel research monitoring wells with data from
nearby existing on-farm drinking water wells. Results
could help to determine whether adequate information concerning
ground water contamination can be obtained from existing
drinking water wells for use in making pollutant control
decisions. The study was conducted during 1993-1994 in
the Little Coharie Watershed, a 158 square mile area located
in the coastal plain of eastern North Carolina. Statistical
analysis indicated that research monitoring wells provided
a greater probability of detecting pesticides in ground
water than existing on-farm wells. Atrazine
was the most frequently detected pesticide found in all
wells, followed in order by fluometuron, carbofuran,
metolachlor, alachlor, carbaryl, butylate, chlorothalonil,
linuron and simazine. Ninety-seven percent of all wells
had observed concentrations of nitrate-N, ranging from
0.1 to 30.1 mg/L. There was not a significant difference
between research wells and existing wells for monitoring
nitrate-N. Based on results of this study, existing drinking
water wells can be used for monitoring nitrate; however,
specialized stainless steel monitoring wells should be
used for monitoring pesticides in ground water.
Ref:
Smith CN el at. (1999). A field study to compare performance
of stainless steel research monitoring wells with existing
on-farm drinking water wells in measuring pesticide and
nitrate concentrations. Chemosphere. Feb;38(4):875-89.
http://www.fluorideaction.org/pesticides/fluometuron.pubmed.htm.
|
|

