|
Return
to Index
Page
Activity: Aerosol
propellant, US EPA List 4B Inert (Halogenated organic)
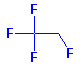
Structure:
Adverse
Effects:
Blood
Body Weight Decrease
Bone
Brain
CNS
Endocrine:
Testicular
Eye
Heart
Kidney
Liver
Lung
Ringing in Ears
Tremors
Environmental
• Note. Approved
for use in the new United States Department of Agriculture
Organic Standards. USDA has approved all List 4 Inerts for
use in organic agriculture.
•
1,1,1,2-Tetrafluoroethane is a
non-ozone depleting alternative to
dichlorodifluoromethane for
use as an air conditioning refrigerant and as a propellant
in anti-asthmatic and other pharmaceutical preparations...
The
atmospheric lifetime of 1,1,1,2-tetrafluoroethane has been
estimated to range from 12.5 to 24 years.
•
Comparative potency studies showed that 1,1,1,2-tetrafluoroethane,
dichlorodifluoromethane, or 1,1,2,2-tetrafluoro-1,2-dichloroethane
(25% gas phase) inhibited gluconeogenesis
about equally while as little as 300 ppm halothane was effective
and 1,1,1,2,2-pentafluoro-2-chloroethane (25%) was without
effect. Considering that the threshold for alteration of
the rate of glucose metabolism in this in vitro paradigm
is about 12.5% 1,1,1,2-tetrafluoroethane, it was concluded
that toxicologically significant alteration of glucose-linked
bioenergetics is unlikely at the levels of 1,1,1,2-tetrafluoroethane
exposure anticipated in workplace or environment. [Olson
MJ et al; Fundam Appl Toxicol 15 (2): 270-80 (1990)]
Ref:
Hazardous Substances Data Bank for 1,1,1,2-TETRAFLUOROETHANE
CASRN: 811-97-2.
http://www.fluorideaction.org/pesticides/1,1,1,2-tetrafluoroe.toxnet.htm
Definition
Gluconeogenesis:
The process of making glucose (sugar) from its own breakdown
products or from the breakdown products of lipids (fats)
or proteins. Gluconeogenesis occurs mainly in cells of the
liver or kidney.
|
Blood
(click
on for all fluorinated pesticides)
HFC-134a (1,1,1,2-Tetrafluoroethane): Subject
#3 was the first volunteer exposed to HFC-134a. The exposure
concentration was 4000 ppm (0.4% v/v) and was scheduled to last
for 30 minutes with a 5-minute postexposure evaluation period
as was accomplished in the Halon 1301 portion of the study. Approximately
4.5 minutes into the exposure, the subject lost consciousness
and both pulse and blood pressure dropped to zero. The
exposure was immediately aborted and the subject was removed from
the exposure apparatus. Medical personnel intervened and after
pulse and blood pressure were restored
the subject was administered 100% oxygen. Blood
pressure and pulse remained low (approximately 1/2 of baseline)
and the subject could not maintain consciousness in a seated position.
The subject was reclined and moved to an operating room recovery
area where he rested for approximately 1 hour after which the
subject's vital signs had returned to pre-exposure values.
Subject #3 displayed a rapid rise in blood concentration of HFC-134a
which reached 1.29 mg/L at the 2.5 minute point in the exposure
(Figure 3). The blood sample scheduled for 3 minutes was not collected.
The medical representative had considerable difficulty getting
blood from the cannula at the 3-minute point and significant manipulation
of the indwelling cannula was noted. No further blood samples
were taken. Subject #5 was also exposed
to 4000 ppm (0.4% v/v) HFC-134a. There was some difficulty
with blood collection and manipulation of the cannula was noted,
but exposure was uneventful through the first 10 minutes of exposure.
Breathing effort and rate appeared normal.
At approximately 10.5 minutes into the exposure the subject's
blood pressure and pulse began to rise rapidly and the subject
gave the hand signal for possible trouble. His
pulse rose rapidly until it was double the pre-exposure value,
at which time the subject gave the hand signal to terminate the
exposure. The exposure
was aborted and the subject began breathing room air, but the
in-dwelling cannula was not removed from the subject. After 30
seconds, the subject's blood pressure and pulse were at pre-exposure
levels. The HFC-134a concentration in blood reached 0.70 mg/L
at the point where the exposure was terminated (Figure 3).
Subject #5 breathed room air for 1 hour and was then re-exposed
to 2000 ppm (0.2% v/v) HFC-134a. After 2.5 minutes of exposure,
the subject's blood pressure and pulse again
rose rapidly, the subject signaled trouble and the exposure was
terminated. The subject's
vital signs returned to pre-exposure levels within 30 seconds
after the exposure was terminated. The in-dwelling cannula remained
attached to the subject and blood was drawn for an additional
10 minutes at 1-minute intervals. The venous blood concentration
of HFC-134a was 0.16 mg/L at the start of the 2000 ppm exposure
and reached 0.38 mg/L at the time of exposure termination (Figure
3). The HFC-134a concentration was still at 0.2 mg/L 10 minutes
after the exposure was terminated. No further human HFC-134a exposures
were conducted. In addition to the monitored effects, there were
several subjective effects associated with the inhalation exposures
to HFC-134a. Subject #3 reported problems
with dizziness and balance following the exposure. At the time
of this report (6 weeks post exposure), both the dizziness and
balance problems still persisted.
Subject #5 reported chest tightness and a headache with associated
dizziness immediately following the exposure. The headache subsided
by the time the subject woke up the day following the exposure.
The day following the exposure, subject #5 reported unusual feelings
in the chest resembling "flutters". The chest tightness was reported
to subside within 3 days of the exposure and the "flutters" within
2 weeks of the exposure. As with subject #3, subject #5 was still
experiencing dizziness and balance problems at the time of this
report (6 weeks post exposure). Subject #5 also reported persistent
ringing in the ears which was still present at the time of this
report. The adverse events observed during the exposures
to HFC-134a and HFC-227ea were unexpected and inconsistent with
the published data. Based on the published
data on HFC-134a and HFC-227ea, no adverse effects should have
been observed at the 0.4% v/v and 0.6% levels, respectively, used
in this study. Both HFC-134a and HFC-227ea have been considered
to be inert compounds which exert toxic effects only after their
concentrations are so high that oxygen depravation effects prevail
(Graepel and Alexander, 1991)...
Rats and mice have shown no acute toxicity during or after a 1-hour
inhalation exposure to 810,000 ppm HFC-134a and dogs were essentially
unaffected following an 80,000 ppm exposure (Alexander,
1995). Based on the laboratory animal data, Alexander,
1995, concluded that HFA-134a is devoid of acute and long
term toxicity, is poorly absorbed and is rapidly excreted. In
addition to the claims of inertness, the chemicals of interest
have been reported to rapidly leave the human system with an apparent
half-life of only 5.1 minutes (Harrison,
1996). Similarly, another report states that only 10% of
the administered dose of HFC-134a remained 10 minutes after termination
of the exposure (Woodcock, 1995).
The 5.1 minute half-life for HFC-134a and extremely rapid elimination
is in contrast to the 31 minute apparent half-life reported as
part of a clinical pharmacology study (Ventresca,
1995). While the sample size was extremely small in our
study due to unplanned termination, the apparent half-lives of
HFC-134a and HFC-227ea are estimated to be 12.6 and 7.5 minutes,
respectively (Figures 2 and 3). This probably represents only
the rapid elimination phase since data were not available to assess
any slower elimination phases that may be present. As such, the
half-life estimates could be quite low especially since
measurable levels of HFC-134a were present 1 hour after
the exposure was terminated (Figure 2). The
presence of HFC-134a in the blood 1 hour after exposure was unexpected.
Alexander, 1995, reported that there was no carry over in blood
after 30 minutes. Halon 1301 cleared more rapidly with an estimated
half-life of 3.6 minutes. Based on published
work, regulatory approval and commercial use of Halon 1301, HFC-134a
and HFC-227ea, the exposure levels selected for the 30-minute
inhalation exposures were expected to be without adverse effects
in humans. Since the study was designed to collect only
kinetic information for use in PBPK model validation and only
at "no effect" concentration levels, clinical type experimental
design was not adopted. Additionally, the subjects participating
in the study were scientists or technicians and they were knowledgeable
about the study results as they occurred... In summary, all 7
human volunteers completed the Halon 1301 exposures without incident
while both the HFC-134a and HFC-227ea exposures were terminated
due to the adverse effects described in this report. Additionally,
no adverse effects were reported during "blank" exposures where
all conditions were the same as in chemical exposures except the
test material was air. Based on the chemical similarity between
HFC-134a and HFC-227ea and the similar human responses during
the exposures, it became the opinion of the investigators that
further exposures would constitute a study of human effects rather
than simply of kinetics. Given this opinion, the study was terminated.
In view of the sample size and experimental design, no conclusion
or speculation about cause and effect is offered at this time.
Rather, the purpose of this document is to report the unexpected
events that occurred during human inhalation of HFC-134a and HFC-227ea
under controlled conditions.
Ref: 1997.
Human Inhalation of Halon 1301, HFC-134a and HFC-227ea for Collection
of Pharmacokinetic Data.
http://www.fluoridealert.org/pesticides/1.1.1.2.3.3.3-Hep.97.REPORT.htm
Body
Weight Decrease
(click
on for all fluorinated pesticides)
-- In a developmental
toxicity study, Lu and Staples (1981)
exposed pregnant CD rats to HFC-134a at 30,000, 100,000, or 300,000
ppm for 6 h/d from days 6 to 15 of gestation. Following exposure
of dams at 300,000 ppm, there was a significant
reduction in fetal weight and significant
increases in several skeletal variations. At 300,000 ppm, signs
of maternal toxicity included reduced food consumption,
reduced body weight gain, lack of
response to noise stimuli, severe tremors,
and uncoordinated movements. Dams exposed at 100,000 ppm
showed reduced response to noise stimuli and uncoordinated movements.
No terata or evidence for developmental toxicity were observed
following exposure of dams at 30,000 or 100,000 ppm.
-- -- Hodge et al. (1979) exposed
groups of 29 or 30 pregnant Wistar-derived rats to HFC-134a at
0, 1,000, 10,000, or 50,000 ppm for 6 h/d on days 6 to 15 of gestation.
Abnormal clinical signs were observed in the animals, but there
was no effect on maternal body weights. At 50,000 ppm, there was
no evidence of terata, but fetal body weight
was significantly reduced, and skeletal
ossification was significantly delayed. There were no effects
on any parameter at 10,000 ppm.
-- -- Groups of 28 pregnant New Zealand white rabbits were exposed
at 0, 2,500, 10,000, or 40,000 ppm for 6 h/d on days 7 through
19 of pregnancy (Collins et al. 1995; Wickramaratne
1989 a,b). Doe were weighed during the study and sacrificed
on day 29 of gestation... In the mid- and high-dose exposure groups,
doe had reduced body weight gains
compared with the control group; lower weight gains were partially
associated with decreased food consumption. With the exception
of a significantly increased incidence of
unossified seventh-lumbar transverse process in fetuses in the
10,000- and 40,000-ppm groups, all other parameters were
similar among control and treatment groups. This effect was also
observed in the control group and was not considered treatment
related. Therefore, there was no adverse developmental or teratogenic
effect associated with exposure to FC-134a.
-- ... Fetotoxicity was ovserved in rats when dams were exposed
at 50,000 ppm (Hodge et al. 1979).
Slight maternal toxicity in rabbits, as indicated by
lower body weight gains compared with the control group,
were noted at 10,000 and 50,000 ppm (Collins
et al. 1995). There was a slight
delay in physical development of F1 rats following exposure
of F0 females at 64,400 ppm (Alexander et
al. 1996).
-- Alexander DJ, Libretto SE, Adams MJ,
Hughes EW, Bannerman M. 1996. HFA-134a (1,1,1,2-tetrafluoroethane):
effects of inhalation exposure upon reproductive performance,
development and maturation of rats. Human Exp Toxicol 15:508-517.
-- Collins MA, Rusch GM, Sato F, Hext PM,
Millischer RJ. 1995. 1,1,1,2-Tetrafluoroethane: repeat exposure
inhalation toxicity in the rat, developmental toxicity in the
rabbit, and genotoxicity in vitro and in vivo. Fundam
Appl Toxicol 25:271-280.
-- Hodge MCE, Kilmartin M, Riley RA, Weight
TM, Wilson J. 1979. Arcton 134a: teratogenicity study in the rat.
ICI Report no. CTL/P/417. Central
Toxicology Laboratory, Alderly Park, Macclesfield, Cheshire, U.K.
Lu M, Staples R. 1981. 1,1,1,2-tetrafkyirietgabe
(FC-134a): embryo-fetal toxicity and teratogenicity study by inhalation
in the rat. Report No. 317-81. Haskell Laboratory,
Wilmington, DE.
-- Wickramaratne GA. 1989a. HCF-134a: Teratogenicity
inhalation study in the rabbit. ICI Report
No. CTL/P/2504. Central Toxicology Laboratory, Alderly Park, Macclesfield,
Cheshire UK (Unpublished).
-- Wickramaratne GA. 1989b. HCF-134a: Embryotoxicity inhalation
study in the rabbit. ICI Report No.
CTL/P/2380. Central Toxicology Laboratory, Alderly Park, Macclesfield,
Cheshire, UK. (Unpublished).
Ref: National Research Council. 2002. Acute
Exposure Guideline Levels for Selected Airborne Chemicals. Volume
2. Subcommittee on Acute Exposure Guideline Levels. Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. National Academy Press, Washington
DC. Available from: National Academy Press, 2101 Constitution
Ave, NW, Box 285, Washington DC 20055. ISBN 0-309-08511-X. Online
at:
http://books.nap.edu/books/030908511X/html/index.html
Bone
(click
on for all fluorinated pesticides)
-- In a developmental
toxicity study, Lu and Staples (1981)
exposed pregnant CD rats to HFC-134a at 30,000, 100,000, or 300,000
ppm for 6 h/d from days 6 to 15 of gestation. Following
exposure of dams at 300,000 ppm, there was a significant reduction
in fetal weight and significant increases
in several skeletal variations. At 300,000 ppm, signs of
maternal toxicity included reduced food consumption,
reduced body weight gain, lack of response to noise stimuli, severe
tremors, and uncoordinated movements. Dams exposed at 100,000
ppm showed reduced response to noise stimuli and uncoordinated
movements. No terata or evidence for developmental toxicity were
observed following exposure of dams at 30,000 or 100,000 ppm.
-- Hodge et al. (1979) exposed groups
of 29 or 30 pregnant Wistar-derived rats to HFC-134a at 0, 1,000,
10,000, or 50,000 ppm for 6 h/d on days 6 to 15 of gestation.
Abnormal clinical signs were observed in the animals, but there
was no effect on maternal body weights. At 50,000 ppm, there was
no evidence of terata, but fetal body weight
was significantly reduced, and skeletal
ossification was significantly delayed. There were no effects
on any parameter at 10,000 ppm.
-- Groups of 28 pregnant New Zealand white rabbits were exposed
at 0, 2,500, 10,000, or 40,000 ppm for 6 h/d on days 7 through
19 of pregnancy (Collins et al. 1995; Wickramaratne
1989 a,b). Doe were weighed during the study and sacrificed
on day 29 of gestation... In the mid- and high-dose exposure groups,
doe had reduced body weight gains compared
with the control group; lower weight gains were partially associated
with decreased food consumption. With the exception of a significantly
increased incidence of unossified seventh-lumbar transverse process
in fetuses in the 10,000- and 40,000-ppm groups, all other
parameters were similar among control and treatment groups. This
effect was also observed in the control group and was not considered
treatment related. Therefore, there was no adverse developmental
or teratogenic effect associated with exposure to FC-134a.
-- -- ... Fetotoxicity was ovserved in rats when dams were exposed
at 50,000 ppm (Hodge et al. 1979).
Slight maternal toxicity in rabbits, as indicated by
lower body weight gains compared with the control group,
were noted at 10,000 and 50,000 ppm (Collins
et al. 1995). There was a slight
delay in physical development of F1 rats following exposure
of F0 females at 64,400 ppm (Alexander et
al. 1996).
-- Alexander DJ, Libretto SE, Adams MJ,
Hughes EW, Bannerman M. 1996. HFA-134a (1,1,1,2-tetrafluoroethane):
effects of inhalation exposure upon reproductive performance,
development and maturation of rats. Human Exp Toxicol 15:508-517.
-- Collins MA, Rusch GM, Sato F, Hext PM, Millischer RJ. 1995.
1,1,1,2-Tetrafluoroethane: repeat exposure inhalation toxicity
in the rat, developmental toxicity in the rabbit, and genotoxicity
in vitro and in vivo. Fundam Appl Toxicol 25:271-280.
-- Hodge MCE, Kilmartin M, Riley RA, Weight
TM, Wilson J. 1979. Arcton 134a: teratogenicity study in the rat.
ICI Report no. CTL/P/417. Central
Toxicology Laboratory, Alderly Park, Macclesfield, Cheshire, U.K.
-- Lu M, Staples R. 1981. 1,1,1,2-tetrafkyirietgabe
(FC-134a): embryo-fetal toxicity and teratogenicity study by inhalation
in the rat. Report No. 317-81. Haskell Laboratory,
Wilmington, DE. (Cited in NRC 1996).
-- Wickramaratne GA. 1989a. HCF-134a: Teratogenicity
inhalation study in the rabbit. ICI Report
No. CTL/P/2504. Central Toxicology Laboratory, Alderly Park, Macclesfield,
Cheshire UK (Unpublished).
-- Wickramaratne GA. 1989b. HCF-134a: Embryotoxicity inhalation
study in the rabbit. ICI Report No.
CTL/P/2380. Central Toxicology Laboratory, Alderly Park, Macclesfield,
Cheshire, UK. (Unpublished).
Ref: National Research Council.
2002. Acute Exposure Guideline Levels for Selected Airborne Chemicals.
Volume 2. Subcommittee on Acute Exposure Guideline Levels, Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. Available from: National Academy
Press, 2101 Constitution Ave, NW, Box 285, Washington DC 20055.
ISBN 0-309-08511-X. Online at:
http://books.nap.edu/books/030908511X/html/index.html
MUSCULOSKELETAL 0.2.15.1 ACUTE EXPOSURE - Rhabdomyolysis
has been reported in a worker susceptible to malignant hyperthermia
after exposure to fluorinated hydrocarbons and also following
intentional freon inhalation. Compartment syndrome is a rare complication
of severe exposure.
Ref: Hazardous Substances
Data Bank for 1,1,1,2-TETRAFLUOROETHANE CASRN: 811-97-2.
http://www.fluorideaction.org/pesticides/1,1,1,2-tetrafluoroe.toxnet.htm
•
Note from FAN: From online site on Rhabdomyolysis
by DR PAUL A. BAGGALEY -----
Rhabdomyolysis is a common disorder which may result from a
large variety of diseases, trauma, or toxic insults to skeletal
muscle. It may be defined as a clinical and biochemical
syndrome resulting from an injury which damages the integrity
of the sarcolemma of skeletal muscle,
leading to the release of potentially toxic muscle cell components
into the circulation.(1,2,3) This may result in potential life-threatening
complications including myoglobinuric acute renal failure, hyperkalaemia
and cardiac arrest, disseminated intravascular coagulation,
and more locally, compartment syndrome.
http://members.tripod.com/~baggas/rhabdo.html
Brain
and CNS
(click
on for all fluorinated pesticides)
-- NEUROLOGIC 0.2.7.1.
ACUTE EXPOSURE - Headache, dizziness, and disorientation are common.
Cerebral edema may be found on autopsy.
A syndrome of impaired psychomotor speed, impaired memory and
learning, and emotional lability has been described in workers
with chronic occupational exposure to fluorinated hydrocarbons.
-- THERE IS A SIGNIFICANT
ACCUMULATION OF FLUOROCARBONS IN BRAIN, LIVER & LUNG COMPARED
TO BLOOD LEVELS, SIGNIFYING A TISSUE DISTRIBUTION OF FLUOROCARBONS
SIMILAR TO THAT OF CHLOROFORM. /FLUOROCARBONS/ [Clayton, G. D.
and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology:
Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons,
1981-1982. 3076]
Ref: Hazardous Substances Data Bank for
1,1,1,2-TETRAFLUOROETHANE CASRN: 811-97-2.
http://www.fluorideaction.org/pesticides/1,1,1,2-tetrafluoroe.toxnet.htm
Endocrine:
Testicular
(click
on for all fluorinated pesticides)
-- groups of 16 male and 16 female rats were exposed at concentrations
of 9, 1000, 10,000, or 50,000 ppm 6 h/d for 20 d of a 28-d period
(Riley et al. 1979). No treatment-related
effects were observed with regard to body weight, clinical signs,
hematology, blood chemistry, urine composition, or ophthalmoscopy.
Changes in liver, kidney, and gonad
weights of male rats
in the group exposed to 50,000 ppm were noted with a significant
increase in liver weight in the 10,000-ppm group also. In the
absence of pathological changes in these organs, Riley
et al. (1979) considered these changes physiological adaptations
to treatment.
-- 3.4 Developmental and Reproductive Toxicity (pages 134 - 136)
-- In a 28-d study conducted by Riley et
al. (1979), 16 male rats were exposed to HFC-134a at 0,
1,000, 10,000, or 50,000 ppm 6 h/d, 5 d/wk. Rats exposed at 50,000
ppm exhibited decreased testicular weights.
However, in a 13-wk study, no effects on testicular weight were
evident (see Section 3.7) (Hext 1989; Collins
et al. 1995). In the chronic study (see Section 3.7) (Collins
et al. 1995), Leydig (interstitial)
cell hyperplasia and benign Leydig cell tumors were reported
following exposure at 50,000 ppm for 104 wk; no such effects were
reported following exposure for 104 wk at 10,000 ppm.
However, it should be noted that these findings are not relevant
for humans because the rat is prone to developing these types
of tumors spontaneously.
-- 3.7 Subchronic and Chronic Toxicity and Carcinogenicity
(pages 138 - 139)
-- groups of 85 male and 85 female rats were exposed to concentrations
at 0, 2,500, 10,000, or 50,000 ppm for 6 h/d, 5 d/wk for 104 wk
(Collins et al. 1995). Exposure conditions
and analytical measurements were identical to procedures followed
in the 13 week study. Ten animals from each group were sacrificed
at 52 wk. At 52 and 104 wk there were no effects on clinical condition,
food consumption, growth, survival, hematology, clinical chemistry,
or urinary parameters. Absolute liver
weights of females were increased
in the groups exposed at 2,500 and 50,000 ppm but not in
the group exposed at 10,000 ppm. Males in groups that received
10,000 or 50,000 ppm for 104 wk had an increased
incidence of enlarges testes (not statistically significant),
and males in the group that received 50,000 ppm for 104 wk had
a statistically significant increase in
incidence of Leydig cell hyperplasia (40 vs. 27 in the
concurrent control group) and Leydig cell
adenomas (23 vs. 9 in the concurrent control group). There
was no evidence of progression to malignancy.
As discussed earlier, these tumors are not
relevant to humans.
-- Although there was an increased
incidence of testicular Leydig cell adenomas in male rats
administered 50,000 ppm for 104 wk (Collins
et al. 1995), these tumors do not progress to malignancy
(Boorman et al. 1990) and
have little significance in humans
(Cook et al. 1999)...
-- Boorman GA, Eustis SL, Elwell MR. 1990.
Pathology of the Fischer Rat: Reference and Atlas. New York Academic
Press, Inc.
-- Riley RA, Bennett IP, Chart IS, Gore CW, Robinson M, Weight
TM. 1979. Arcton-134a: Subacute toxicity to the rat by inhalation.
ICI Report No. CTL/P/463. Central Toxicology Laboratory,
Alderly Park, Macclesfield, Cheshire, UK.
-- Collins MA, Rusch GM, Sato F, Hext PM, Millischer RJ. 1995.
1,1,1,2-Tetrafluoroethane: repeat exposure inhalation toxicity
in the rat, developmental toxicity in the rabbit, and genotoxicity
in vitro and in vivo. Fundam Appl Toxicol 25:271-280.
-- Cook JC, Klinefelter GR, Hardisty JF, Sharpe RM, Foster PMD.
1999. Rodent Leydig cell tumorigenesis: a review of the physiology,
pathology, mechanisms, and relevance to humans. Crit Rev Toxicol
29:169-261.
-- Hext PM. 1989. 90-day inhalation toxicity study in the rat.
ICI Report No. CTL/P/2466. Central
Toxicology Laboratory, Imperial Chemical Industries, Alderley
Park, Macclesfield, Cheshire, U.K. (Cited in NRC 1996).
Ref: National Research Council. 2002.
Acute Exposure Guideline Levels for Selected Airborne Chemicals.
Volume 2. Subcommittee on Acute Exposure Guideline Levels. Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. National Academy Press, Washington
DC. Available from: National Academy Press, 2101 Constitution
Ave, NW, Box 285, Washington DC 20055. ISBN 0-309-08511-X . Online
at:
http://books.nap.edu/books/030908511X/html/index.html
Eye
(click
on for all fluorinated pesticides)
-- In the perinatal and postnatal part of the study, groups of
41 female rats were administered concentrations of 1,800, 9,900,
or 64,400 ppm of HFC-134a (99.3% pure) for 1 h daily during days
17 to 20 of pregnancy and days 1 to 21 postpartum (Alexander
et al. 1996). Females were allowed to deliver and rear
their young. Selected F1 animals were mated; these animals were
sacrified on day 20 of pregnancy, and the uterine contents were
examed... There was a statistically significant
delay in the occurrence of pinnae detatchment,
eye-opening, and startle response
in the F1 generation, whose dams inhaled 64,400 ppm....
-- Alexander DJ, Libretto SE, Adams MJ,
Hughes EW, Bannerman M. 1996. HFA-134a (1,1,1,2-tetrafluoroethane):
effects of inhalation exposure upon reproductive performance,
development and maturation of rats. Human Exp Toxicol 15:508-517.
Ref: National Research Council. 2002. Acute
Exposure Guideline Levels for Selected Airborne Chemicals. Volume
2. Subcommittee on Acute Exposure Guideline Levels, Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. Available from: National Academy
Press, 2101 Constitution Ave, NW, Box 285, Washington DC 20055.
ISBN 0-309-08511-X. Online at:
http://books.nap.edu/books/030908511X/html/index.html
Heart
(click
on for all fluorinated pesticides)
4.2 Mechanism of Toxicity (pages 143 - 144)
At high concentrations, HFC-134a has anesthetic and narcotic properties;
cardiac sensitization may also occur.
The biochemical mechanism(s) of action of these two efects is
not well understood. The anesthetic effect was fully reversible.
Inhalation of certain hydrocarbons, including some anesthetics,
can make the mammalian heart abnormally
sensitive to epinephrine, resulting in ventricular arrhythmias,
which is some cases can lead to sudden death (Reinhardt
et al. 1971). The mechanism of action of cardiac sensitization
is not completely understood but appears to involve a disturbance
in the normal conduction of the electrical impulse through the
heart, probably by producing a local disturbance in the electrical
potential across cell membranes. The hydrocarbons themselves do
not produce arrhythmia; the arrhythmia
is the result of the potentiation of endogenous epinephrine (adrenalin)
by the hydrocarbon ... In the study with human volunteers exposed
to HFC-134a (Emmen and Hoogendijk 1998),
the relationship between exposure concentration and blood level
was linear, and at all exposure concentrations (1,000, 2,000,
4,000, and 8,000 ppm), blood concentrations approached equilibrium
at 55 min. Cardiac sensitization is considered
a concentration threshold phenomenon (page 146).
-- Emmen HH, Hoogendijk EMG. 1998. Report
on an ascending dose safety study comparing HFA-13a with CFC-12
and air, administered by whole-body exposure to healthy volunteers.
MA-250B-82-306, TNO Report V98.754, The Netherlands Organization
Nutrition and Food Research Institute, Zeist, The Netherlands.
-- Reinhardt CF, Azar A, Maxfield ME, Smith
PE, Mullin LS. 1971. Cardiac arrhythmias and aerosol "sniffing."
Arch Environ Health 22:265-279.
Ref: National Research Council. 2002. Acute
Exposure Guideline Levels for Selected Airborne Chemicals. Volume
2. Subcommittee on Acute Exposure Guideline Levels. Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. National Academy Press, Washington
DC. Available from: National Academy Press, 2101 Constitution
Ave, NW, Box 285, Washington DC 20055. ISBN 0-309-08511-X. Online
at:
http://books.nap.edu/books/030908511X/html/index.html
Subject #3 was the first volunteer
exposed to HFC-134a. The exposure concentration was 4000 ppm (0.4%
v/v) and was scheduled to last for 30 minutes with a 5-minute
postexposure evaluation period as was accomplished in the Halon
1301 portion of the study. Approximately
4.5 minutes into the exposure, the subject lost consciousness
and both pulse and blood pressure dropped to zero. The
exposure was immediately aborted and the subject was removed from
the exposure apparatus. Medical personnel intervened and after
pulse and blood pressure were restored the subject was administered
100% oxygen. Blood pressure and pulse remained
low (approximately 1/2 of baseline) and the subject could not
maintain consciousness in a seated position. The subject
was reclined and moved to an operating room recovery area where
he rested for approximately 1 hour after which the subject's vital
signs had returned to pre-exposure values...
Subject #5 was also exposed
to 4000 ppm (0.4% v/v) HFC-134a. There was some difficulty with
blood collection and manipulation of the cannula was noted,
but exposure was uneventful through the first 10 minutes of exposure.
Breathing effort and rate appeared normal. At
approximately 10.5 minutes into the exposure the subject's blood
pressure and pulse began to rise
rapidly and the subject gave the hand signal for possible trouble.
His pulse rose rapidly until it was double the pre-exposure value,
at which time the subject gave the hand signal to terminate the
exposure. The exposure was aborted and the subject began
breathing room air, but the in-dwelling cannula was not removed
from the subject. After 30 seconds, the subject's blood pressure
and pulse were at pre-exposure levels.
The HFC-134a concentration in blood reached 0.70 mg/L at the point
where the exposure was terminated (Figure 3).
Subject #5 breathed room air for 1 hour
and was then re-exposed to 2000 ppm (0.2% v/v) HFC-134a. After
2.5 minutes of exposure, the subject's
blood pressure and pulse again rose rapidly, the subject signaled
trouble and the exposure was terminated.
The subject's vital signs returned to pre-exposure levels
within 30 seconds after the exposure was terminated... No further
human HFC-134a exposures were conducted. In addition to the monitored
effects, there were several subjective effects associated with
the inhalation exposures to HFC-134a. Subject
#3 reported problems with dizziness and balance following the
exposure. At the time of this report (6 weeks post exposure),
both the dizziness and balance problems still persisted. Subject
#5 reported chest tightness and a headache with associated dizziness
immediately following the exposure. The headache subsided by the
time the subject woke up the day following the exposure.
The day following the exposure, subject
#5 reported unusual feelings in the chest resembling "flutters".
The chest tightness was reported to subside within 3 days of the
exposure and the "flutters" within 2 weeks of the exposure.
As with subject #3, subject #5 was still experiencing dizziness
and balance problems at the time of this report (6 weeks post
exposure). Subject #5 also reported persistent ringing in the
ears which was still present at the time of this report. The
adverse events observed during the exposures to HFC-134a and HFC-227ea
were unexpected and inconsistent with the published data...
Based on published work, regulatory approval and commercial use
of Halon 1301, HFC-134a and HFC-227ea, the exposure levels selected
for the 30-minute inhalation exposures were expected to be without
adverse effects in humans.
Ref: 1997.
Human Inhalation of Halon 1301, HFC-134a and HFC-227ea for Collection
of Pharmacokinetic Data.
http://www.fluoridealert.org/pesticides/1.1.1.2.3.3.3-Hep.97.REPORT.htm
Kidney
(click
on for all fluorinated pesticides)
Comparative potency studies showed that 1,1,1,2-tetrafluoroethane,
dichlorodifluoromethane, or 1,1,2,2-tetrafluoro-1,2-dichloroethane
(25% gas phase) inhibited gluconeogenesis
about equally while as little as 300 ppm halothane was effective
and 1,1,1,2,2-pentafluoro-2-chloroethane (25%) was without effect.
Considering that the threshold for alteration of the rate of glucose
metabolism in this in vitro paradigm is about 12.5% 1,1,1,2-tetrafluoroethane,
it was concluded that toxicologically significant alteration of
glucose-linked bioenergetics is unlikely at the levels of 1,1,1,2-tetrafluoroethane
exposure anticipated in workplace or environment. [Olson MJ et
al; Fundam Appl Toxicol 15 (2): 270-80 (1990)]
Ref: Hazardous Substances Data Bank for 1,1,1,2-TETRAFLUOROETHANE
CASRN: 811-97-2.
http://www.fluorideaction.org/pesticides/1,1,1,2-tetrafluoroe.toxnet.htm
Note from FAN:
Definition Gluconeogenesis: The process of making glucose (sugar)
from its own breakdown products or from the breakdown products
of lipids (fats) or proteins. Gluconeogenesis occurs mainly
in cells of the liver or kidney.
-- groups of 16 male and 16 female rats were exposed at concentrations
of 9, 1000, 10,000, or 50,000 ppm 6 h/d for 20 d of a 28-d period
(Riley et al. 1979). No treatment-related
effects were observed with regard to body weight, clinical signs,
hematology, blood chemistry, urine composition, or ophthalmoscopy.
Changes in liver,
kidney, and gonad weights of male
rats in the group exposed
to 50,000 ppm were noted with a significant increase in liver
weight in the 10,000-ppm group also. In the absence of pathological
changes in these organs, Riley et al. (1979)
considered these changes physiological adaptations to treatment.
-- Riley RA, Bennett IP, Chart IS, Gore
CW, Robinson M, Weight TM. 1979. Arcton-134a: Subacute toxicity
to the rat by inhalation. ICI Report
No. CTL/P/463. Central Toxicology Laboratory, Alderly Park, Macclesfield,
Cheshire, UK.
Ref: National Research Council. 2002. Acute
Exposure Guideline Levels for Selected Airborne Chemicals. Volume
2. Subcommittee on Acute Exposure Guideline Levels. Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. National Academy Press, Washington
DC. Available from: National Academy Press, 2101 Constitution
Ave, NW, Box 285, Washington DC 20055. ISBN 0-309-08511-X. Online
at:
http://books.nap.edu/books/030908511X/html/index.html
Liver
(click
on for all fluorinated pesticides)
Comparative potency studies showed that 1,1,1,2-tetrafluoroethane,
dichlorodifluoromethane, or 1,1,2,2-tetrafluoro-1,2-dichloroethane
(25% gas phase) inhibited gluconeogenesis
about equally while as little as 300 ppm halothane was effective
and 1,1,1,2,2-pentafluoro-2-chloroethane (25%) was without effect.
Considering that the threshold for alteration of the rate of glucose
metabolism in this in vitro paradigm is about 12.5% 1,1,1,2-tetrafluoroethane,
it was concluded that toxicologically significant alteration of
glucose-linked bioenergetics is unlikely at the levels of 1,1,1,2-tetrafluoroethane
exposure anticipated in workplace or environment. [Olson MJ et
al; Fundam Appl Toxicol 15 (2): 270-80 (1990)]
Note from FAN: Definition
Gluconeogenesis: The process of making glucose (sugar) from its
own breakdown products or from the breakdown products of lipids
(fats) or proteins. Gluconeogenesis occurs mainly in cells of
the liver or kidney.
Ref: Hazardous Substances Data Bank for 1,1,1,2-TETRAFLUOROETHANE
CASRN: 811-97-2.
http://www.fluorideaction.org/pesticides/1,1,1,2-tetrafluoroe.toxnet.htm
-- groups of 16 male and 16 female rats were exposed at concentrations
of 9, 1000, 10,000, or 50,000 ppm 6 h/d for 20 d of a 28-d period
(Riley et al. 1979). No treatment-related
effects were observed with regard to body weight, clinical signs,
hematology, blood chemistry, urine composition, or ophthalmoscopy.
Changes in liver, kidney, and gonad
weights of male rats in the group exposed to 50,000 ppm
were noted with a significant increase in
liver weight in the 10,000-ppm group also. In the absence
of pathological changes in these organs, Riley
et al. (1979) considered these changes physiological adaptations
to treatment.
-- 3.7 Subchronic and Chronic Toxicity and Carcinogenicity (pages
138 - 139)
... groups of 85 male and 85 female rats were exposed to concentrations
at 0, 2,500, 10,000, or 50,000 ppm for 6 h/d, 5 d/wk for 104 wk
(Collins et al. 1995). Exposure conditions
and analytical measurements were identical to procedures followed
in the 13 week study. Ten animals from each group were sacrificed
at 52 wk. At 52 and 104 wk there were no effects on clinical condition,
food consumption, growth, survival, hematology, clinical chemistry,
or urinary parameters. Absolute liver weights
of females were increased in the groups exposed at 2,500
and 50,000 ppm but not in the group exposed at 10,000 ppm. Males
in groups that received 10,000 or 50,000 ppm for 104 wk had an
increased incidence of enlarges testes (not
statistically significant), and males in the group that received
50,000 ppm for 104 wk had a statistically significant increase
in incidence of Leydig cell hyperplasia (40 vs. 27 in the concurrent
control group) and Leydig cell adenomas (23 vs. 9 in the
concurrent control group). There was no evidence of progression
to malignancy. As discussed earlier, these
tumors are not relevant to humans.
-- Collins MA, Rusch GM, Sato F, Hext PM,
Millischer RJ. 1995. 1,1,1,2-Tetrafluoroethane: repeat exposure
inhalation toxicity in the rat, developmental toxicity in the
rabbit, and genotoxicity in vitro and in vivo. Fundam
Appl Toxicol 25:271-280.
-- Riley RA, Bennett IP, Chart IS, Gore
CW, Robinson M, Weight TM. 1979. Arcton-134a: Subacute toxicity
to the rat by inhalation. ICI Report
No. CTL/P/463. Central Toxicology Laboratory, Alderly Park, Macclesfield,
Cheshire, UK.
Ref: National Research Council. 2002. Acute
Exposure Guideline Levels for Selected Airborne Chemicals. Volume
2. Subcommittee on Acute Exposure Guideline Levels. Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. National Academy Press, Washington
DC. Available from: National Academy Press, 2101 Constitution
Ave, NW, Box 285, Washington DC 20055. ISBN 0-309-08511-X. Online
at:
http://books.nap.edu/books/030908511X/html/index.html
Lung
(click
on for all fluorinated pesticides)
3.2.3 Rats (pages 131 - 132). Groups
of ten male rats were exposed at concentrations of 0, 10,000,
50,000, or 100,000 ppm for 6 h/d, 5 d/wk for 2 wk (Silber
and Kenedy 1979b). Five rats from each group were sacrificed
at the end of the tenth exposure, and the remaining fie rats per
group were sacrificed after a 14-d recovery period. No treatment-related
changes in weight gain, hematology parameters, blood chemistry,
or organ weights were observed. Increased incidence of focal
interstitial pneumonitis of the lung was the only adverse
effect observed in the groups exposed at 50,000 and 100,000
ppm. The fluoride content of the urine was significantly increased
in the treated rats.
-- Silber LS, Kennedy GL. 1979b. Subacute
inhalation toxicity of tetrafluoroethane (FC 134a). Haskell
Laboratory, Report No. 422-79, DuPont
de Nemours and Company, Newark DE, cited in ECETOC, 1995.
Ref: National Research Council. 2002. Acute
Exposure Guideline Levels for Selected Airborne Chemicals. Volume
2. Subcommittee on Acute Exposure Guideline Levels, Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. Available from: National Academy
Press, 2101 Constitution Ave, NW, Box 285, Washington DC 20055.
ISBN 0-309-08511-X. Online at:
http://books.nap.edu/books/030908511X/html/index.html
Ringing
in Ears (click
on for all fluorinated pesticides)
-- The adverse events observed during the exposures to HFC-134a
and HFC-227ea were unexpected and inconsistent with the published
data. Based on the published data on HFC-134a and HFC-227ea, no
adverse effects should have been observed at the 0.4% v/v and
0.6% levels, respectively, used in this study.
-- Subject #3 reported problems with dizziness and balance following
the exposure. At the time of this report (6 weeks post exposure),
both the dizziness and balance problems still persisted. Subject
#5 reported chest tightness and a headache with associated dizziness
immediately following the exposure. The headache subsided by the
time the subject woke up the day following the exposure. The day
following the exposure, subject #5 reported unusual feelings in
the chest resembling "flutters". The chest tightness was reported
to subside within 3 days of the exposure and the "flutters" within
2 weeks of the exposure. As with subject #3, subject #5 was still
experiencing dizziness and balance problems at the time of this
report (6 weeks post exposure). Subject #5 also reported persistent
ringing in the ears which was still
present at the time of this report.
-- The presence of HFC-134a in the blood 1 hour after exposure
was unexpected.
-- In summary, all 7 human volunteers completed the Halon 1301
exposures without incident while both the HFC-134a and HFC-227ea
exposures were terminated due to the adverse effects described
in this report.
Ref: 1997. Human Inhalation of Halon 1301,
HFC-134a and HFC-227ea for Collection of Pharmacokinetic Data.
19 Aug 97.
http://www.fluoridealert.org/pesticides/1.1.1.2.3.3.3-Hep.97.REPORT.htm
Tremors
(click
on for all fluorinated pesticides)
-- In a developmental toxicity study, Lu
and Staples (1981) exposed pregnant CD rats to HFC-134a
at 30,000, 100,000, or 300,000 ppm for 6 h/d from days 6 to 15
of gestation. Following exposure of dams at 300,000 ppm, there
was a significant reduction in fetal weight
and significant increases in several skeletal variations. At 300,000
ppm, signs of maternal toxicity included
reduced food consumption, reduced body weight gain, lack of response
to noise stimuli, severe tremors,
and uncoordinated movements. Dams exposed at 100,000 ppm showed
reduced response to noise stimuli and uncoordinated movements.
No terata or evidence for developmental toxicity were observed
following exposure of dams at 30,000 or 100,000 ppm.
-- Lu M, Staples R. 1981. 1,1,1,2-tetrafkyirietgabe
(FC-134a): embryo-fetal toxicity and teratogenicity study by inhalation
in the rat. Report No. 317-81. Haskell Laboratory,
Wilmington, DE.
Ref: National Research Council. 2002. Acute
Exposure Guideline Levels for Selected Airborne Chemicals. Volume
2. Subcommittee on Acute Exposure Guideline Levels, Committee
on Toxicology, Board of Environmental Studies and Toxicology,
Division of Earth and Life Studies. Available from: National Academy
Press, 2101 Constitution Ave, NW, Box 285, Washington DC 20055.
ISBN 0-309-08511-X. Online at:
http://books.nap.edu/books/030908511X/html/index.html
| Environmental
(click
on for all fluorinated pesticides)
The
atmospheric lifetime of 1,1,1,2-tetrafluoroethane has been
estimated to range from 12.5 to 24 years.
1,1,1,2-Tetrafluoroethane may also undergo atmospheric removal
by wet deposition processes; however, any removed is expected
to rapidly re-volatilize to the atmosphere.
Ref: Hazardous
Substances Data Bank for 1,1,1,2-TETRAFLUOROETHANE CASRN:
811-97-2.
http://www.fluorideaction.org/pesticides/1,1,1,2-tetrafluoroe.toxnet.htm
|
|

