http://www.startribune.com/stories/1556/4927444.html
August 15, 2004
Star Tribune (Minneapolis
- St. Paul, Minnesotta)
Former 3M chemical
is widespread
By David Shaffer
Four years after 3M stopped making the chemicals behind such innovative
products as Teflon, Scotchgard and Stainmaster, the compounds are showing
up everywhere from remote Minnesota lakes to polar bears in Alaska and
albatrosses in the Pacific Ocean.
The compounds, known as fluorochemicals, are a concern because they don't
degrade and it's unclear how they spread. The U.S. Environmental Protection
Agency (EPA) is assessing whether one of the compounds poses health or
environmental risks, but it does not consider the fluorochemicals harmful.
A steady stream of
scientific studies in the past six years has reported low levels of the
compounds in humans, supermarket foods and wildlife all over the world,
including fish in the Great Lakes.
Now, in a study that
underscores the pervasiveness of fluorochemicals, EPA-funded
researchers at the University of Minnesota say they have detected traces
of the compounds in two remote lakes in the Voyageurs National Park near
International Falls, Minn. One lake is 11 miles from the nearest road.
Tracing chemicals around the world
3M, based in Maplewood,
was a leading manufacturer of the compounds until 2000, when it stopped
making, selling and using them -- and it reformulated Scotchgard -- because
the compounds were so widespread. One of its big customers had been DuPont
Co., which still uses a fluorochemical to make Teflon and other coatings.
Last week, DuPont denied charges by the EPA that for two decades it withheld
health information that it was required to submit about one of the chemicals.
The EPA is seeking fines that could total millions of dollars.
The study of Minnesota lakes was led by Matt Simcik,
an assistant professor of environmental chemistry, who presented preliminary
results at a scientific conference in May. Simcik said researchers also
found the chemicals in two lakes in Tettegouche State Park near Silver
Bay, Minn., in Lake Michigan and in the Minneapolis Chain of Lakes.
Researchers
at the University of Iowa recently detected fluorochemicals in lakes Ontario
and Erie. The
study, published in June in the journal Environmental Science & Technology,
said traces of the chemicals were found in all 16 samples taken.
"They are not
high in a toxic sense," said Keri Hornbuckle, an associate professor
of civil and environmental engineering at the University of Iowa. "There
is no danger from exposure to the water, but they
are high enough that they there is no question that animals are going
to accumulate them from the aquatic food chain."
Hornbuckle said fluorochemicals
are different from other persistent -- and highly toxic -- substances
found widely in the environment. They include the banned pesticide DDT,
the banned industrial oil/coolant mixture known as PCBs and mercury, a
toxic element released by coal-burning power plants.
The spread of fluorochemicals
is partly the result of consumer demand for stain-repellent carpeting,
breathable waterproof jackets and nonstick cooking utensils. "We
like these chemicals to keep the water off our back and to keep food from
sticking on our pots," Hornbuckle said. "They are the result
of our wealth."
Some studies have
suggested that 90 percent of Americans, including children, have the chemicals
at low levels in their bodies. Yet scientists have not determined how
they got into people.
A related mystery
is how these chemicals are transported in the environment. Simcik said
air currents may spread them, which would explain how they got into remote
lakes. Another possibility, said Hornbuckle, is that the chemicals are
washing off the stain-resistant or waterproof fabrics and ending up in
wastewater discharges.
Decades old
Few people other than
scientists have heard of these chemicals, which carry the almost unpronounceable
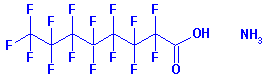
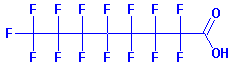
names perfluorooctanoic acid (PFOA) and perfluorooctane sulfate (PFOS).
In 1945, chemical
maker Du Pont of Wilmington, Del., introduced Teflon, a product that over
the next five decades would become a household name. Its use spread far
beyond cookware to industries ranging from aerospace to computers. DuPont
says such products can't be made without the chemical PFOA.
Seven years after
Teflon's debut, Patsy Sherman, a chemist fresh out of college, was working
for 3M in Minnesota on a substance to coat aircraft tires when a lab mishap
helped launch another highly successful fluorochemical product -- Scotchgard
fabric protector.
"I had made a
sample of one of the fluorochemical rubbers," said Sherman, who retired
in 1992 and now lives in Bloomington. "Another person to whom I had
given it dropped it. It splashed it over brand-new tennis shoes. We tried
to remove the fluorochemical using all the solvents we could find. Nothing
would even wet these stains."
Many of the Scotchgard
stain-repelling products relied on fluorochemical cousins of PFOS. For
decades, 3M produced fluorochemicals at its plant in Cottage Grove and
also in Decatur, Ala. 3M turned away from the chemicals, and ended
production at the plants, in 2000. DuPont, which still needed PFOA for
its coatings business, built its own manufacturing plant in North Carolina.
Since at least the
mid-1970s, scientists have known that fluorochemicals end up in humans'
blood. 3M's medical director Larry Zobel said the company began regularly
testing fluorochemical workers in 1976 and has consistently found the
chemical in their blood. Over the years, "we found no adverse health
effects in our workers," he added.
The workers
were told of the results, 3M says.
Recent findings that
three fluorochemical workers in a 3M Alabama plant died of bladder
cancer -- a higher number than normally would be expected -- are
being studied further, Zobel said. Production workers have 50 to 500 times
more of the chemicals in their blood than the general population, he added.
The knowledge in the
1970s that fluorochemicals end up in human blood did not trigger the kind
of EPA review that has been taking place since last year. The process,
called a risk assessment, could lead to regulations of PFOA.
EPA charges
DuPont
The EPA last month
charged that DuPont repeatedly failed to pass information about PFOA to
the government, including data on the company's 1981 monitoring of pregnant
workers at a West Virginia plant and the discovery of the chemical in
one child's umbilical cord. The EPA is seeking millions of dollars in
fines.
In a response filed
Wednesday, DuPont said it wasn't required to submit the information and
asked for a hearing. It said that PFOA found in the umbilical cord did
not harm the baby. DuPont also is facing a class-action lawsuit by residents
of a West Virginia community where PFOA has been detected in the water
supply.
Chris Lagan, an EPA
spokesman, said the DuPont information could have triggered an earlier
EPA inquiry into the chemical. "Had we had that information, is it
reasonable to say we would have acted sooner?" he said. "It
is possible it [the current risk assessment] would have happened then."
3M has not been accused
by the EPA of withholding information about the chemicals. The Minnesota
Health Department said its tests of wells near 3M's Cottage Grove plant
found no fluorochemicals.
Zobel of 3M said testing
equipment improved in the 1990s, leading to the wave of reports by company
researchers and others that fluorochemicals are widespread. The evidence
prompted 3M to stop making and using the compounds, said Michael Santoro,
3M's director of regulatory affairs.
The expanding knowledge
about fluorochemicals in the environment raises the question of how they
could spread so widely before being comprehensively studied by federal
regulators.
"We don't have
a forward-looking system," said Timothy Kropp, senior scientist with
the Environmental Working Group, a Washington, D.C., organization that
has published EPA, 3M and DuPont documents on its Web site. "We have
a backward-looking system. Something has to be a problem for EPA to look
at it."
David Shaffer is at
dshaffer@startribune.com.