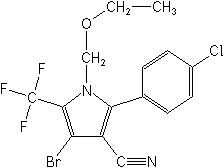
Chlorfenapyr
4-bromo-2-(4- chlorophenyl)-1-(ethoxymethyl)-5-(trifluoromethyl)-1H-pyrrole-3- carbonitrile
August 11, 2003, comments submitted to US EPA on the pesticide
petition from BASF Corporation to establish a tolerance for residues of chlorfenapyr
on all food items in food handling establishments where food products
are held, processed, and/or prepared at 0.01 parts per million (ppm).
Petition published in the
Federal Register, July 16, 2003.
Docket ID Number OPP-2003-0205
Submitted by
Ellen Connett
Fluoride Action Network Pesticide Project
82 Judson Street, Canton, NY 13617
Tel: 315-379-9200
Email: wastenot@northnet.orgAugust 11, 2003
To: US EPA Office of Pesticide Programs
Re: Submission to Docket ID number OPP-2003-0205
Via: Email: opp-docket@epa.gov
1.
In animal studies, Chlorfenapyr induced spongiform encephalopathy in mice and spongiform myelopathy in mice and rats.1.1
In a subchronic oral toxicity study in mice (MRID 43492830) chlorfenapyr technical was administered to mice at dietary dose levels of 0, 40, 80, 160, or 320 ppm (average 0, 7.1, 14.8, 27.6, or 62.6 mg/kg/day, respectively, for males; 0, 9.2, 19.3, 40.0, or 78.0 mg/kg/day, respectively, for females) for 91 days... Spongiform encephalopathy was noted in the brain and myelin of the spinal cord of both males and females receiving the 320 ppm treatment level...
Ref: US EPA OPPT. February 12, 1998. SUBJECT: Chlorfenapyr - 129093: Health Effects Division Risk Characterization for Use of the Chemical Chlorfenapyr (Alert, EPA File Symbol 5905-GAI) in/on Citrus (6F04623). Case: 287132. Barcode: D221320- http://www.epa.gov/opprd001/chlorfenapyr/memohed2.pdf1.2
SUBCHRONIC STUDIES ** 010, 031; 125163; "AC 303,630: A 13 Week Dietary Toxicity Study in the Albino Rat. Histopathology: spongiform myelopathy in brain, spinal cord (M-(2/20), 1200, 900 ppm, (1/20), 600 ppm), lesion present in sciatic nerve (M-(1/20), 1200 ppm), lymphoid cell infiltrate in kidneys (M,F-900, 1200 ppm); Target organ: central nervous system; Adverse Effect: spongiform myelopathy in the nervous system; NOEL: (M) 300 ppm (occurance of spongiform myelopathy in the nervous system of the 600 ppm group)
Ref: August 24, 2001 - Summary of Toxicological Data. California EPA. Department of Pesticide Regulation. Medical Toxicology Branch. Also available at: http://www.cdpr.ca.gov/docs/toxsums/pdfs/3938.pdf1.3
Information at the website of the University of Leicester for Prion Diseases states:Prion diseases are often called spongiform encephalopathies because of the post mortem appearance of the brain with large vacuoles in the cortex and cerebellum. Probably most mammalian species develop these diseases. Specific examples include:
• Scrapie: sheep
• TME (transmissible mink encephalopathy): mink
• CWD (chronic wasting disease): muledeer, elk
• BSE (bovine spongiform encephalopathy): cowsEvidence suggests that a prion is a modified form of a normal cellular protein known as PrPc (for cellular)... It has been proposed that PrPsc when introduced into a normal cell causes the conversion of PrPc into PrPsc. The exact nature of the process is unknown but it could involve a chemical or conformational modification.
Ref http://www-micro.msb.le.ac.uk/3035/prions.html1.4
The hallmarks of prion diseases are
-- spongiform change
-- neuronal loss
-- astrocyte proliferation1.5
Is chlorfenapyr a chemical that induces prion diseases? If not, could the US EPA please explain why.2.
More studies on chlorfenapyr need to be done:2.1.
The US EPA needs to replicate the MRID 43492830 mouse study (cited in 1.1) to ascertain if the spongiform encephalopathy induced by Chlorfenapyr is transmissable. Studies need to be conducted by intracerebral inoculation of homogenate of mice brain from chlorfenapyr-induced spongiform encephalopathy into mice and rat brain. Also, the brains of at least two generations of animals from chlorfenapyr-induced spongiform encephalopathy and spongiform myelopathy should be studied.2.2
If US EPA decides not to conduct these studies, then they should provide a convincing explanation to the public as to why the Chlorfenapyr-induced spongiform encephalopathy would not be transmissable to mice or other species, or to other generations of mice from parents analysed with Chlorfenapy-induced spongiform encephalopathy.2.3
The formula for chlorfenapyr should be altered by removing the bromine. Government scientists should repeat the MRID 43492830 mouse study to determine if spongiform encephalopathy is induced without the bromine.2.4.
The same as above (2.3), but to remove the fluorine.2.5
Such an experiment may lead to an understanding of the effects of fluorine and bromine on the brain. It may also resolve the question of what is inducing the observed spongiform encephalopathy. Is it
-- the combination of fluorine plus bromine in the chlorfenapyr formula
-- the fluorine alone
-- the bromine alone3.
US EPA should provide the public with more information to allow an informed discussion and decision making process in regards to this petition.3.1
US EPA should provide data to the public of all pesticides, and chemicals used in pesticidal formulations, known to produce spongiform encephalopathy and spongiform myelopathy. For example, is chlorfenapyr the only pesticide known to induce spongiform encephalopathy?3.2
Due to the growing number of people diagnosed with neurodegenerative diseases, US EPA should provide the public with its rationale for allowing the manufacture and use of pesticides that have the potential to induce spongiform encephalopathy and spongiform myelopathy.3.3
US EPA should perform a health risk assessment of the workers involved in the production of chlorfenapyr;3.4
US EPA should provide a risk assessment and worst-case scenarios for an accidental release into the communities where chlorfenapyr is produced.3.5
US EPA should provide an explanation as to why male mice were more sensitive to chlorfenapyr-induced spongiform myelopathy.4.
Has the developmental neurotoxicity study for chlorfenapyr, recommended in 1998, been performed? The recommendation was:The RfD Committee also recommended that a special developmental neurotoxicity study be conducted based upon the effects of a spongyform myelopathy and/or vacuolation seen in the brain and spinal cord of treated rats and mice. They concluded that the registrant should also conduct a mechanistic study to determine the cause/relationship of CNS/myelinopathic alterations to neurotoxicity (including developmental). The Ad Hoc Committee considered the following modifications to the developmental neurotoxicity study protocol are necessary: A 90 day treatment period for males and females prior to the routine developmental phase required in the developmental neurotoxicity study guidelines is needed. The dams would deliver their pups and come off treated feed at day 10 post-delivery. Normal testing as required in the developmental neurotoxicity study guidelines would then commence. Further, the Ad Hoc Committee and the Toxicology Branch considered it necessary to characterize the nature of the vacuoles reported in the previous studies and any found in the presently proposed study. The treated males would be used to assist in this characterization. This information may play a role in assessing the potential risk of this chemical. It is strongly recommended that the registrant contact the HED prior to initiating the study in order to discuss dose selection and study protocol. It should be noted that the Registrant has requested modifications to the protocol of the neurotoxicity study. This request is currently under consideration by HED.
Ref: US EPA OPPT. February 12, 1998. SUBJECT: Chlorfenapyr - 129093: Health Effects Division Risk Characterization for Use of the Chemical Chlorfenapyr (Alert, EPA File Symbol 5905-GAI) in/on Citrus (6F04623). Case: 287132. Barcode: D221320- http://www.epa.gov/opprd001/chlorfenapyr/memohed2.pdf4.1
Would US EPA explain the modifications to the protocol of the neurotoxicity study requested by the Registrant.4.2
If the study recommended by the RfD Committee was performed, it should have been included in the "documents" in the Docket for this petition. Why were no documents available to the public via the Docket?5.
Endocrine Disruption. In the petition, BASF states:... There is no information available which suggests that chlorfenapyr would be associated with endocrine effects.
5.1
However, US EPA noted endocrine effects in the following study:In the rat chronic toxicity/carcinogenicity study (MRID 43492837), there were increased trends in the incidence of hepatocellular adenomas, hepatocellular adenomas and/or carcinomas combined, malignant histiocytic sarcomas and testicular interstitial cell tumors in males rats. In female rats there were significant increasing trends in endometrial stromal polyps. Significant difference in pair-wise comparison of fibroadenomas at the low dose and carcinomas at the mid-dose existed for female rats. There was no evidence of tumorigenic potential in mice....
Ref: US EPA OPPT. February 12, 1998. SUBJECT: Chlorfenapyr - 129093: Health Effects Division Risk Characterization for Use of the Chemical Chlorfenapyr (Alert, EPA File Symbol 5905-GAI) in/on Citrus (6F04623). Case: 287132. Barcode: D221320- http://www.epa.gov/opprd001/chlorfenapyr/memohed2.pdf5.2
The study cited above (MRID 43492837) appears to contradict BASF's assertion that "... There is no information available which suggests that chlorfenapyr would be associated with endocrine effects."6.
There are only two pesticides that I have found with both bromine and fluorine as active ingredients. One is chlorfenapyr and the other is the rodenticide bromethalin. Both are highly toxic to the brain. Results from some bromethalin animals studies cited by US EPA are:--spongy degeneration (leukoencephalomyelopathy) observed in most of the central white fiber tracts of the rat brain, cerebellum, pons, brain stem, and thoracic spinal cord of both sexes and optic nerves of males (MRID 43582102).
-- Subchronic toxicity study: Beagle dogs. spongy degeneration observed in nervous tissue components (cervical, thoracic, and lumbar spinal cord, brain stem, right and left optic nerves, frontal and median brain, pons, and cerebellum) (MRID 43582101).
-- Dogs given a single oral dose of bromethalin at 6.25 mg/kg. Histologic lesions included diffuse white matter spongiosis, mild microgliosis, and optic nerve vacuolization. Ultramicroscopic examination of brainstem revealed occasional swollen axons, intramyelenic vacuolization, and myelin splitting at the intraperiod line."
Ref: US EPA Reregistration Eligibility Decision (RED) Rodenticide Cluster. EPA738-R-98-007. July 1998.
http://www.fluoridealert.org/pesticides/Bromethalin.RED.EPA..1998.pdf6.1
Is EPA aware of any other pesticides where the active ingredient, or the inerts used in a pesticidal formulation, contain both fluorine and bromine?7.
US EPA should provide the public an explanation of the interaction of chlorfenapyr with other pesticides known to impact the brain. For example, does the potential exist for more severe brain effects if a consumer is exposed simultaneously to both, such as exposure to the following organofluorine pesticides?7.1
There are several organofluorine pesticides that in animal studies have been found to adversely affect the brain. Due to time constraints, the following is a limited list.7.2 SULFURYL FLUORIDE - Fumigant - Fluorine insecticide
7.2.1 Sulfuryl fluoride should be of some interest to EPA because it has been touted as the alternative fumigant to methyl bromide. Dow AgroSciences has petitioned US EPA for tolerances on more than 40 raw and processed food commodities.
Ref: Federal Register: February 15, 2002. Notice of Filing a Pesticide Petition to Establish a Tolerance for a Certain Pesticide Chemical in or on Food. http://www.epa.gov/fedrgstr/EPA-PEST/2002/February/Day-15/p3661.htm7.2.2 On Feb 7, 2002, US EPA in a Final Rule approved a 3-year Experimental Use Permit for inorganic fluoride residues in or on raisin at 30 ppm and in or on walnut at 12 ppm. However, this Final Rule is being challenged, and according to EPA it is not being implemented.
Ref: Federal Register, Final Rule. http://www.epa.gov/fedrgstr/EPA-PEST/2002/February/Day-07/p2983.htm7.2.3 In animal studies, Sulfuryl fluoride was found to adversely affect the brain:
-- In rabbits, the primary target organ was the brain, in which malacia (necrosis) and vacuolation were observed in the cerebrum...
-- In subchronic (90-day) inhalation studies in rats, dogs, rabbits and mice, the brain was the major target organ. Malacia and/or vacuolation were observed in the white matter of the brain in all four species. The portions of the brain most often affected were the caudate-putamen nucleus in the basal ganglia, the white fiber tracts in the internal and external capsules, and the globus pallidus of the cerebrum...
-- In chronic (1-2 year) inhalation studies in rats, dogs and mice, target organs were the same as in the 90-day studies... . Other treatment-related effects in rats included effects in the brain (vacuolation of the cerebrum and thalamus/hypothalamus)... In dogs and mice, increased mortalities, malacia and/or vacuolation in the white matter in the brain, histopathology in the lungs, and follicular cell hypertrophy in the thyroid gland were observed...
-- In a 2-generation reproduction inhalation study in rats, vacuolation of the white matter in the brain...
Ref: Federal Register. September 5, 2001. Sulfuryl Fluoride; Proposed Pesticide Temporary Tolerances.
http://www.fluoridealert.org/pesticides/Sulfuryl.Flu.FR.Sept.5.2001.htm7.3
FLUAZINAM - Fungicide. Molecular formula: C13H4Cl2F6N4O4
7.3.1 Fluazinam: Males: 555 mg/kg/day; Females: 658 mg/kg/day based on vacuolation of white matter in brain, increased liver weights, histopathology in liver...
Eight special mechanistic studies to assess the CNS white matter vacuolation: White matter vacuolation in the CNS of mice, rats, and dogs was found to be due to Impurity-5.
Ref: Federal Register. September 7, 2001. Fluazinam; Pesticide Tolerance. Final Rule.
http://www.fluoridealert.org/pesticides/Fluazinam.FR.Sept.7.2001.htm7.3.2 Fluazinam: US EPA has approved the Establishment of an import tolerance for residues of fluazinam and its metabolite in or on wine grapes 3.0 ppm, and peanuts at 0.02 ppm.
Ref: Federal Register: April 18, 2002, Fluazinam; Pesticide Tolerance. Final Rule.7.3.3 Fluazinam: EPA approved a tolerrance of 0.02 ppm on potatoes.
Ref: Federal Register: September 7, 2001. Fluazinam; Pesticide Tolerance. Final Rule. http://www.epa.gov/fedrgstr/EPA-PEST/2001/September/Day-07/p22525.htm7.4
FLUFENACET - Herbicide. Molecular formula: C14H13F4N3O2S7.4.1
Flufenacet: NOEL = 40 ppm [1.29 mg/kg/day in males and 1.14 mg/kg/day in females] LOEL = 800 ppm [27.75 mg/kg/day in males and 26.82 mg/kg/day in females] based on increased alkaline phosphatase, kidney, and liver weight in both sexes, increased cholesterol in males, decreased T3, T4 and ALT values in both sexes, and increased incidence of microscopic lesions in the brain [axonal degeneration], eye [vacuolization of the ciliary body epithelium], kidney [hyperplasia of the epithelial cells], spinal cord [axonal degeneration], sciatic nerve [axonal degeneration] and liver [hepatocytomegaly].
Ref: EPA Pesticide Fact Sheet, April 1998. http://www.epa.gov/opprd001/factsheets/flufenacet.pdf7.4.2
Flufenacet: Currently, US EPA has 36 food tolerances. The highest is for Wheat, forage at 10 ppm.7.5
SODIUM FLUORIDE - EPA List 4 Inert - Fluorine insecticide7.5.1
In a long-term, low dose rat study, 1 ppm Sodium fluroide was administered in doubly distilled drinking water. The authors concluded: "In summary, chronic administration of AlF3 and NaF in the drinking water of rats resulted in distinct morphological alterations in the brains, including effects on neurons and cerebrovasculature."
Ref: Varner JA et al. (1998). Chronic administration of aluminum-fluoride and sodium-fluoride to rats in drinking water: alterations in neuronal and cerebrovascular integrity. Brain Research, 784:284-298.7.6
TEFLUTHRIN - Insecticide - Molecular Formula: C17H14Cl F7 O2
7.6.1
Tefluthrin: In a 3-month rat study, dietary administration of 10 mg/kg/day produced plasma, red blood cell, and brain cholinesterase inhibition. The NOEL was 5 mg/kg/day. In a 6-month dog study, dietary administration of 10 mg/kg/day (LOEL) produced plasma cholinesterase inhibition. The NOEL was 1 mg/kg/day.
Ref: USEPA/OPP. Support Document for the Addition of Chemicals from Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active Ingredients to EPCRA Section 313. U. S. Environmental Protection Agency, Washington, DC (1993). As cited by US EPA in: Federal Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition of Certain Chemicals; Toxic Chemical Release Reporting; Community Right-to-Know; Proposed Rule.7.6.2
Tefluthrin. Currently US EPA has 18 food tolerances for corn at 0.06 ppm.7.7
ACIFLUORFEN. Herbicide - Molecular formula: C14H7Cl F3 NO57.7.1 Acifluorfen: ... the Hazard Identification Assessment Review Committee recommended that a developmental neurotoxicity study in rats be conducted based on neurotoxicity observed in a developmental toxicity study in rats (increased incidence of dilated lateral ventricles of the fetal brain, MRID 00122743). In addition, no neurotoxicity studies are available for acifluorfen or for structurally related compounds which might provide an understanding on the effects of acifluorfen on the developing nervous system.
Ref: EPA, Sodium Acifluorfen. HED Chapter for the Reregistration Eligibility Decision. April 27, 2001. http://www.epa.gov/oppsrrd1/reregistration/acifluorfen/newrisk.pdf7.7.2 Currently US EPA has 60 food tolerances for Aciflurfen.
7.8
DFP: Diisopropyl fluorophosphate - Former Insecticide. Molecular formula: C6H14FO3P
-- While this pesticide is no longer used, the effects on the brain are of some interest --7.8.1
DFP. In a study of alkyl phosphate poisoning, Pasi and Leuzinger came to the conclusion that delayed lesions only occur, if at all, after severe cerebral anoxia [176]. As regards anatomical changes in the brain (demyelination), these delayed lesions correspond to those caused by peripheral neuropathy in acute and chronic ortho-tricresyl phosphate poisoning and are confined to fluorine- containing alkyl phosphates—for example, mipafox [formerly used as an insecticide], DFP, sarin and soman. ..
Ref: Delayed Toxic Effects of Chemical Warfare Agents. A SIPRI (Stockholm international Peace Research Institute) Monograph. 1975. ISBN 91-85114-29-4. http://projects.sipri.se/cbw/research/cw-delayed.pdf8
Has EPA considered the cumulative effects of exposure to various brain-toxic pesticides on fetal brain development?9.
Has US EPA considered the effects of chlorfenapyr on other susceptible subsets of the population with neurodegenerative diseases such as
-- Parkinson's
-- Multiple Sclerosis
-- HIV-1 Associated Vacuolar Myelopathy9.1
Has US EPA considered the subsets of the population who are exposed to chlorfenapyr through imported food?
For example, tea drinkers. Japan has MRL's for chlorfenapyr for several agricultural products. Of concern is the very high tolerance of 50 ppm for Tea (Green, Black, Oolong, and Wulung).
Ref: Japan's Pesticide Standard Limits -- Downloaded August 5, 2003. http://www.ffcr.or.jp/zaidan/psl.nsf/88e391f9074a0ca54925663d001a0caa/57d76a2a15fd3f51492566940019d667?OpenDocument10.
Can Chlorfenapyr be safely disposed? Can US EPA explain how.10.1
As the petition requests a tolerance for residues "on all food items in food handling establishments where food products are held, processed, and/or prepared at 0.01 parts per million (ppm)", can these food products be safely
-- composted
-- fed to farm animals or pets
-- fed to birds10.2
What are the transformation products when chlorfenapyr is incinerated?
11.
What are the "inerts" used in Chlorfenapyr pesticide products? If US EPA doesn't provide this information, how can they expect the public to contribute anything substantive when information on the majority of the chlorfenapyr formulation is withheld? I urge the EPA to divulge the names of the chemicals, and their percentages, used in chlorfenapyr pesticide products. I also urge US EPA to revisit the "Inerts" issue.