http://www.aist.go.jp/aist_e/latest_research/2006/20060314/20060314.html
Japan’s National Institute for Advanced Industrial Science
and Technology (AIST)
March 14, 2006 (Translation of the AIST press release of February
21, 2006)
Efficient Decomposition of Environmentally
Persistent and Bioaccumulative Organofluorine Compound "PFOS"
by Use of Sub-Critical Water with Iron
Key Points
1. Perfluorooctane sulfonate (PFOS),
a highly bioaccumulative organofluorine compound, can
be effectively decomposed to fluoride ions by
use of water and iron.
2. The high stability (difficulty of
decomposition) of organofluorine compounds hinders their waste
treatment. In particular, PFOS is highly stable and there
have been no effective methods for its decomposition, except
for incinerating at high temperature.
3. Further studies will be conducted
to the decomposition and recycling of organofluorine compounds
of larger molecular weights.
Summary
National Institute for Advanced Industrial Science and Technology
(AIST, Hiroyuki Yoshikawa, President) has succeeded in the development
of an efficient method to decompose environmentally persistent
and bioaccumulative perfluorooctane sulfonate (PFOS) and related
compounds to fluoride ions.
Organofluorine compounds have been widely used in many industries
as surfactants because they have excellent properties such as
heat and chemical resistance, light transparency, etc. However,
some of them show high environmental persistence and bioaccumulation
so that the development of effective waste treatment methods is
desired. PFOS is has been globally detected in environmental waters
and wildlife and its long-term toxicity (negative health effects
caused by continued intake of the substance) is suspected. PFOS
shows very high chemical and thermal stability; it cannot be decomposed
even if it is boiled in sulfuric acid.
AIST has achieved highly efficient decomposition of PFOS into
fluoride ions by adding iron powder to water containing PFOS and
taking the sample to sub-critical water state at 250-350°C.
The produced fluoride ions may be recycled as a source of fluorine
using an established processing method for fluoride ions. This
method was successfully applied to decompose other related fluorochemicals
and to decompose PFOS contained in a coating agent used in electronic
industry.
Details of this method were published in
the February issue (No. 3) of Environmental Science & Technology,
Vol. 40, published by the American Chemical Society.
Background
Organofluorine compounds have unique characteristics (repulsion
to water and oil, resistance to heat and to chemical substances,
non-absorption of light) so that they have been used as surfactants
such as surface treatment agents, emulsifiers, coatings, etc.
However, it has been recently reported that some of them are persist
in the environment and accumulate in wildlife. PFOS is a representative
example. Because of the environmental persistence and bioaccumulation
of PFOS, the United States Environmental Protection Agency (USEPA)
established regulations controlling the use of PFOS in April 2002,
and in November of the same year, the Organization for Economic
Cooperation and Development (OECD) published a hazard assessment
of PFOS. In December of the same year, PFOS became a designated
compound according to the Chemical Substances Control Law (currently
class II specified chemical substance) in Japan. In June of 2005,
PFOS was nominated at the Stockholm Convention, where international
regulations are being examined.
Therefore, it is extensively desired to develop effective waste
treatment techniques for PFOS and related compounds with low energy
costs.
Details of Research Work
AIST has been conducting research on the analytical methods,
environmental fate, and decomposition methods for organofluorine
compounds.
Description of Research Work
The outline of this decomposition method is as follows. A stainless
steel reactor (volume 34.3 mL) containing an aqueous solution
of PFOS (10 mL, PFOS concentration: 46-186 ppm) and iron powder
(0.54 g) or other metal powders, was introduced in an argon atmosphere.
Then, the temperature was raised to the sub-critical state at
250-350°C.
After a certain time, the reactor was cooled to room temperature
and the components in the reactor were analyzed. A reaction without
metal powder was also performed. When no metal powder was added,
little PFOS was decomposed. The highest decomposition of PFOS
was achieved when iron powder was used.
For example, when an initial PFOS concentration was 186 ppm and
the reaction temperature was 350°C [pressure in the reactor
was 23.3 MPa (1 MPa = 9.87 atmospheric pressure)], PFOS completely
disappeared in water after six hours of the treatment, and at
the same time, fluoride ions efficiently produced. The PFOS decomposition
occurred on the iron surface. After six hours of the treatment,
organofluorine compounds (PFOS or its reaction intermediates)
were still detected on the surface of iron. However, after prolonged
treatment, all organofluorine species on the surface disappeared.
This method was also effective to the decomposition of shorter-chain
related fluorochemicals (perfluoroaklyl sulfonates) and was successfully
applied to the decomposition of PFOS contained in an a coating
agent used in electronic industry.
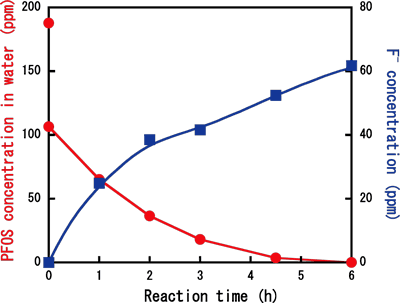
Figure
Decrease in PFOS concentration and increase in fluoride-ion concentration
by sub-critical water treatment with iron powder. Initial concentration
of PFOS: 186 ppm, Reaction temperature: 350°C, Reaction pressure:
23.3 MPa [Reprinted with permission from Environmental Science
& Technology, 2006, 40, 1049-1054. Copyright 2006. American
Chemical Society].
Copyright(C) National Institute of Advanced Industrial Science
and Technology (AIST). All rights reserved.