• Click here to
return to the Lung section
for fluorine & organofluorine pesticides.
Excerpts
of The
Toxicology of Perfluoroisobutene by Jiri Patocka and
Jiri Bajgar.
Perfluoroisobutene
... is a fluoro-olefin produced by thermal decomposition
of polytetrafluoroethylene (PTFE), e.g., Teflon [1].
Overheating
of PTFE generates fumes of highly toxic PFIB and poses
a serious health hazard to the human respiratory tract.
PFIB is approximately ten times
as toxic as phosgene [2]. Inhalation of this gas
can cause pulmonary edema, which can lead to death.
PFIB is included in Schedule 2 of the Chemical Weapons
Convention (CWC), as a result of the prompting
by one delegation to the Conference on Disarmament [3].
...
Acute
Toxicity. The median lethal concentration (LC50) in single
exposures of rats was 0.5 ppm. The intoxicated rats either
died with gross pathological signs
of pulmonary congestion or recovered with no apparent
residual effects. The 15-second LC50 was 361 ppm and the
10-minute LC50 was 17 ppm [9]. Similar high acute toxicity
following inhalation was seen in other species with a
two hour LC50 in mice reported to be either 1.6 ppm [9]
or 0.98 ppm [10], in rabbits either 4.3 ppm [10] or 1.2
ppm [11], in guinea-pigs 1.05 ppm [10] and in cats 3.1
ppm [10]. In experiments in which rats were exposed to
a concentration of 12.2 ppm for 10 min, an unusual postexposure
latency period of approximately 8 hours was observed prior
to the occurrence of pulmonary edema
[12].
Human
Toxicity ... Five workers accidentally exposed to a gas
containing 2 percent PFIB reported irritation of the respiratory
tract within 24 h of exposure. The
lung irritation was manifested by cough in all cases.
The patients developed headache, cough, substernal
pain, dyspnoea and fever within the first hour following
exposure. The symptoms became worse at six to eight hours
after exposure. Also choking or
shortness of breath was observed in a majority of the
patients. The condition of the patients began to
improve on the fifth day and they were discharged from
the hospital after two to three weeks. One patient developed
a respiratory infection, which required his stay in hospital
to be extended to about eight weeks. All the patients
were shown to have pulmonary edema
and this was confirmed at post mortem on two patients
died. One died after 11 days after
exposure, the other died after 13 days [16, 17].
According to Kennedy [14], one worker exposed to PFIB
for three minutes reported strong subjective symptoms
of bad odor, bad taste in the mouth, nausea and weakness.
On returning to fresh air, the worker recovered and had
no further symptoms. Half an hour later, a concentration
of 0.04 ppm of PFIB was measured in the exposure area.
The latency period for PFIB injury is one to four hours
until pulmonary edema symptoms appear. In many cases,
pulmonary edema clears up in about 72 hours, with little
long-term damage [27].
Pathology
The histopathology of rat lung
has been studied after an acute exposure to PFIB at a
concentration of 78 ppm for 1.5 min. Within 5 min of exposure,
changes to the bronchioles and peribronchial
alveoli were observed which took the form of alterations
to cilial structure, increased pinocytosis with occasional
vesicle formation of type I alveolar epithelial cells.
The gradual development of pulmonary edema was
visible histologically two to three hours post exposure,
with death occurring from seven hours onwards. Animals
sacrificed at 24 hours post exposure showed evidence of
widespread pulmonary edema and alveolar
interstitial infiltration by lympho mononuclear cells
and macrophages [19]. In other experiments, PFIB
induced pulmonary edema involving a translocation of blood
compartment proteins into the lung’s alveolar compartment.
By high-performance capillary electrophoresis of proteins
from the fluid lining of the lungs of rats exposed to
PFIB was estimated that albumin, transferrin and IgG are
three major proteins translocated into the alveolar space
[20].
References
1. Zeifman, Y.B., Ter-Gabrielyan, N.P., Knunyants, I.L.
The Chemistry of Perfluoroisobutylene. Uspekhi Khimii,
1984; 53: 431-461.
2. Oberdorster, G., Ferin, J., Gelein, J., Finkelstein,
R., Baggs, R., Effects of PTFE Fumes in the Respiratory
Tract: A Particle Effect? Aerospace Medical Assiciation
65th Annual Scientific Meeting, 1994; 538: A52.
3. CD/CW/WP.239. Verification of the Nonproduction of
Chemical Weapons: An Illustrative Example of the Problem
of Novel Toxic Chemicals. 12 April 1989.
9. Smith, L.W., Gardner, R.J., Kennedy, G.L., Jr. Short-Term
Inhalation Toxicity of Perfluoroisobutylene. Drug Chem
Toxicol 1982; 5: 295-303.
14. Kennedy, G.L., Jr., Geisen, R.J. Setting Occupational
Exposure Limits for Perfluoroisobutylene, A Highly Toxic
Chemical Following Acute Exposure. J Occup Med 1985; 27:
675-.
16. Unpublished data from DuPont Co., cited as Reference
15.
17. Danishevskii, S.L., Kochanov, M.M. Toxicity of Some
Fluoroorganic Compounds. Gig Tr Prof Zabol 1961; 5: 3-8.
19. Brown, R.F., Rice, P. Electron Microscopy of Rat Lung
Following a Single Acute Exposure to Perfluoroisobutylene
(PFIB). A Sequential Study of the First 24 hours Following
Exposure. Int J Exp Pathol 1991; 72: 437-450.
20. Gurley, L.R., Buchanan, J.S., London, J.E., Stavert,
D.M., Lehnert, B.E. High Performance Capillary Electrophoresis
of Proteins from the Fluid Lining of the Lungs of Rats
Exposed to Perfluoroisobutylene. J Chromatogr 1991; 559:
411-429.
Ref:
Toxicology of Perfluoroisobutene by Jiri Patocka and Jiri
Bajgar (Department of Toxicology, Military Medical Academy
500 01 Hradec, Czech Republic). The ASA Newsletter (Applied
Science and Analysis, Inc.). 1998.
http://www.asanltr.com/ASANews-98/pfib.html
|
Oxygen
difluoride
is a thermal decomposition product
of Teflon.
It has many synonyms; it's
primary name is
Fluorine monoxide
(CAS No. 7783-41-7).
The
molecular structure is:
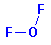
In
the US National Institute for Occupational Safety and
Health (NIOSH) category of
Immediately
Dangerous to Life or Health Concentrations (IDLH)
-
a list of 387 workplace chemicals - the
two chemicals that share the rank of most dangerous chemicals
for "respirator selection criteria" are:
Oxygen
difluoride : IDLH of 0.5 ppm
Lithium hydride : IDLH of 0.5 mg/m3
Human
data: Oxygen
difluoride is a strong irritant
to the entire respiratory tract and causes pulmonary edema
and hemorrhage when inhaled for a few hours at 0.5 ppm
[Deichmann and Gerarde 1969].
Basis
for original (SCP) IDLH: The
chosen IDLH is based on the statements by Deichmann and
Gerarde [1969] that oxygen
difluoride is a strong irritant to the entire respiratory
tract and causes pulmonary edema and hemorrhage when inhaled
for a few hours at 0.5 ppm. Development of
pulmonary signs leading to death may be delayed several
hours after the exposure [Deichmann and Gerarde 1969].
In addition, AIHA [1967] reported that the Committee
on Toxicology of the National Research Council recommended
an Emergency Exposure Limit (EEL)
of 0.5 ppm for a 10-minute exposure. This
EEL is supposed to be for exposures that are "rare
in the lifetime of an individual and permit some degree
of reversible injury short of incapacitation" [Smyth 1966].
ACUTE
TOXICITY DATA:
Lethal concentration data: |
Species
|
Reference |
LC50
(ppm) |
Time |
Adjusted
0.5-hr LC (CF) |
Derived
value |
| Rat |
Darmer
et al. 1972 |
2.6 |
1
hr |
3.3
ppm (1.25) |
0.3
ppm |
| Mouse |
Darmer
et al. 1972 |
1.5 |
1
hr |
1.9
ppm (1.25) |
0.2
ppm |
| Dog |
Darmer
et al. 1972 |
26 |
1
hr |
33
ppm (1.25) |
3.3
ppm |
| Monkey |
Darmer
et al. 1972 |
16 |
1
hr |
20
ppm (1.25) |
2.0
ppm |
References:
AIHA
[1967]. Oxygen difluoride. In: Hygienic guide series.
Am Ind Hyg Assoc J 28:194-196.
Darmer
KI Jr, Haum CC, MacEwen JD [1972]. The acute inhalation
toxicology of chlorine pentafluoride. Am Ind Hyg Assoc
J 33:661-668.
Deichmann
WB, Gerarde HW [1969]. Oxygen difluoride (OF2). In: Toxicology
of drugs and chemicals. New York, NY: Academic Press,
Inc., p. 444.
Smyth
HF Jr [1966]. Military and space short-term inhalation
standards. Arch Environ Health 12:488-490.
|
Abstract: When Teflon is heated the developing fumes produce
in exposed humans an influenza-like syndrome
(polymer fume fever) or also
severe toxic effects like pulmonary edema,
pneumonitis and death. The decomposition products and the
resulting health effects are temperature-dependent. The toxic
effects seem to be related to the ultrafine particulate fraction
of the fume. To test the hypothesis that exposure to ultrafine
particles results in an increased interstitialization of the particles
which is accompanied by an acute pathological inflammation, rats
were exposed to titanium dioxide (TiO2) particles by intratracheal
instillation and by inhalation. Both acute intratracheal instillation
and subchronic inhalation studies on rats show that ultrafine
TiO2 particles (~20 nm diameter) access the pulmonary interstitium
to a larger extent than fine particles (~250 nm diameter) and
that they elicit an inflammatory response as indicated by PMN
increase in lavaged cells. The release of ultrafine particles
into the air of an enclosed environment from a thermodegradation
event or from other sources is a potential hazard for astronauts.
Knowing the mechanisms of action is a prerequisite for technical
or medical countermeasures.
Ref: Part 2—Biomedical support. Polymer
degradation and ultrafine particles: Potential inhalation hazards
for astronauts. by J. Ferin and G. Oberdörster (Environmental
Health Sciences Center School of Medicine & Dentistry University
of Rochester, Rochester, NY 14642, USA). Acta Astronautica Volume
27 , July 1992, Pages 257-259.
Abstract: The effect of exposure to polytetrafluoroethylene (9002-84-0)
(PTFE) fumes on the early pulmonary inflammatory response was
examined. Male Fischer-344-rats
were exposed to PTFE fumes produced at 460 degrees-C. A
particle concentration of 5x10(5) particles per cubic centimeter
was determined. Controls were sham exposed. Animals were sacrificed
4 hours after exposure and pulmonary lavage was performed. The
messenger RNA (mRNA) in lung tissue was analyzed by Northern and
slot blot analysis. Membrane hybridization and in-situ hybridization
were conducted. The mean percentage of neutrophils (PMNs) in the
lavage fluid increased from 0.55% in controls to 55.7% in exposed
rats. The mean percentage of lymphocytes increased from 0.5% in
controls to 2.64% in exposed rats. The mean
percentage of macrophages decreased from 98.9% in controls to
41.6% in exposed rats. Compared to controls, the levels
of protein, lactate-dehydrogenase, and beta-glucuronidase in the
lavage fluid of rats exposed to PTFE fumes were elevated by 35,
7, and 6.5 fold, respectively. The
quantity of mRNAs for various antioxidants
and cytokines increased by up to 40 fold, compared to controls,
following PTFE exposure. In-situ hybridization revealed
that the transcripts of interleukin-6 (IL6), metallothionein (MT),
and tumor necrosis factor alpha (TNF-alpha) were not elevated
in the cells of control lungs. In lungs exposed to PTFE fumes,
the transcripts of MT and IL6 were elevated in lymphatic vessels,
large and small airways, inflammatory cells, parenchyma cells,
and where pulmonary edema was evident. Following exposure, TNF-alpha
transcripts were elevated in the parenchyma and interstitium,
whereas the transcripts of surfactant protein-C were increased
in type-II cells, the interstitium, and surrounding airways. The
authors conclude that exposure to PTFE fumes containing ultrafine
particles induces an early pulmonary inflammatory response.
This inflammatory process may be mediated by PMNs and the up regulation
of cytokines and antioxidants.
Ref: Characterization of the Early
Pulmonary Inflammatory Response Associated with PTFE Fume Exposure;
by Johnston CJ, Finkelstein JN, Gelein R, Baggs R, Oberdorster
G. Toxicology and Applied Pharmacology,
Vol. 140, No. 1, pages 154-163, 1996.
Abstract: PTFE
(polytetrafluoroethylene) fumes consisting of large numbers of
ultrafine (uf) particles and low concentrations of gas-phase compounds
can cause severe acute lung injury.
Our studies were designed to test three hypotheses:
(i) uf PTFE fume particles are causally
involved in the induction of acute lung injury,
(ii) uf PTFE elicit greater pulmonary effects
than larger sized PTFE accumulation mode particles, and
(iii) preexposure to the uf PTFE fume particles will induce tolerance.
We used uf Teflon (PTFE) fumes (count median particle size ~ 16
nm) generated by heating PTFE in a tube furnace to 486°C to
evaluate principles of ultrafine particle toxicity.
Teflon fumes at ultrafine particle concentrations
of 50 g/m3 were extremely toxic to rats when inhaled for only
15 min. We found that when generated in argon, the ultrafine
Teflon particles alone are not toxic at these exposure conditions;
neither were Teflon fume gas-phase constituents when generated
in air. Only the combination of both phases
when generated in air caused high toxicity, suggesting
either the existence of radicals on the surface or a carrier mechanism
of the ultrafine particles for adsorbed gas compounds. Aging of
the fresh Teflon fumes for 3.5 min led to a predicted coagulation
to >100 nm particles which no longer caused toxicity in exposed
animals. This result is consistent with a greater toxicity of
ultrafine particles compared to accumulation mode particles, although
changes in particle surface chemistry during the aging process
may have contributed to the diminished toxicity. Furthermore,
the pulmonary toxicity of the ultrafine Teflon fumes could be
prevented by adapting the animals with short 5-min exposures on
3 days prior to a 15-min exposure. Messages encoding antioxidants
and chemokines were increased substantially in nonadapted animals,
yet were unaltered in adapted animals. This study shows the importance
of preexposure history for the susceptibility to acute ultrafine
particle effects.
Ref: Pulmonary Effects Induced by Ultrafine PTFE Particles; Carl
J. Johnston, Jacob N. Finkelstein, Pamela Mercer, Nancy Corson,
Robert Gelein and Günter Oberdörster. Toxicology and
Applied Pharmacology Volume 168, Issue 3 , 1 November 2000, Pages
208-215.
Abstract: The cases of three patients with acute pulmonary oedema
caused by inhalation of fumes from heated polytetrafluoroethylene
(PTFE) in a plastic factory are described.
One patient died from profound hypoxemia
and shock shortly after admission, and the other two patients
survived after medical treatment. This is the first report of
fatal pulmonary oedema in
a worker exposed to PTFE heated in a plastic extruding operation.
From this observation, it appears that inhalation exposure
to pyrolytic products from polytetrafluoroethylene can cause fatal
respiratory complications. Special precautions are warranted in
this kind of operation to prevent workers from being exposed to
these substances.
Ref: Fatal acute pulmonary oedema after
inhalation of fumes from polytetrafluoroethylene (PTFE) by LEE
CH, GUO YL, TSAI PJ, CHANG HY, CHEN CR, CHEN CW, HSIUE T-R. EUROPEAN
RESPIRATORY JOURNAL; 10 (6). 1997. 1408-1411.
Abstract: Information
on potential occupational hazards from exposure to carbonyl fluoride
(353-50-4) was reviewed. Topics discussed included chemical and
physical properties, production, use, manufacturers and distributors,
manufacturing processes, occupational exposure, and biological
effects. Potential
exposure to carbonyl fluoride occurs as a result
of the thermal decomposition of polytetrafluoroethylene (PTFE)
in air. Effects of acute exposure in animal studies included
extreme malaise and weakness which preceded
death. Subchronic exposure
studies with PTFE pyrolysis products revealed pathologic
changes in the respiratory tracts and livers of exposed animals.
Protein, glucose, ketones, and occult blood appeared in
the urine following exposure. No information was available concerning
chronic exposures, carcinogenicity, mutagenicity, teratogenicity,
or reproductive effects.
Ref:
1987. Information Profiles on Potential Occupational Hazards:
Carbonyl Fluoride. Second Draft. Syracuse Research Corp., NY.
Center for Chemical Hazard Assessment. Sponsored by National Inst.
for Occupational Safety and Health, Rockville, MD. Report No.
NTIS/PB87-174330.
Abstract.
Toxic effects following inhalation exposure to polytetrafluoroethylene
(9002-84-0) (PTFE) pyrolysis products were determined in rats.
Greenacres-Flora-rats were exposed to PTFE pyrolysis products
containing hydrolyzable fluoride equal to 50 parts per million
of carbonyl fluoride (353-50-4) for 1 hour daily for 5 days. On
day 1 and 5 of the exposure period, and 3, 7, and 18 days postexposure
urine samples were collected and examined for fluoride excretion
and glucose, protein, and ketones. On each of those days, a test
animal was killed, and kidney and lung tissues were tested for
succinic-dehydrogenase activity. Weight changes and mortality
during the course of the experiment were also noted. During the
5 exposure days and shortly afterwards, mortality reached 22 percent,
although the total exposure dose was less than half the median
lethal dose for one exposure. Daily urinary
fluoride excretion jumped to 14 times normal on the first exposure
day and remained at 4 times normal by the eighteenth postexposure
day. By
the fifth exposure day, body weights dropped 30 percent, urine
glucose, protein, and ketones were abnormal, and succinic-dehydrogenase
activity dropped to near zero in the kidney and had more than
doubled in the lung;
by the eighteenth post exposure day, these values had returned
to normal. The authors conclude that carbonyl
fluoride generated during the pyrolysis of PTFE hydrolyzes in
body fluids and produces fluoride toxicity. The
cumulative effect of repeated exposures is much more toxic than
a single equivalent exposure. If death does not result, the metabolic
inhibition due to fluoride poisoning is completely reversible.
Ref:
Biochemical Changes Associated with Toxic Exposures to Polytetrafluoroethylene
Pyrolysis Products by Scheel LD, McMillan L, Phipps FC. American
Industrial Hygiene Association Journal, Vol. 29, No. 1, pages
49-53, 1968.
Abstract: 1990
NTIS Report: Perfluoroisobutylene (PFIB) is an extremely toxic
organofluoride that can be produced during pyrolysis of tetrafluoroethylene
polymers, including Teflon.
Inhalation of PFIB at very low concentrations
causes acute lung injury, the hallmark of which is pulmonary edema.
Several lines of evidence have suggested that hydrolysis
of PFIB and resulting production of hydrofluoric acid may be responsible
for pulmonary damage. In order to investigate the potential involvement
of hydrofluoric acid in producing lung injury and its relationship
to the mechanism of fluorocarbon toxicity, we have compared the
pulmonary injury produced by PFIB, by dissociated (H(sup +) and
F(sup (minus))), and by undissociated (HF) hydrofluoric acid in
the deep lung. By delivering hydrofluoric acid by intratracheal
instillation in neutral buffer, we demonstrate that F(sup (minus))
produces no significant pulmonary injury as assessed by increased
in lung weight and ultrastructural changes. Similarly, instillation
of aci [abstract truncated]
Ref:
1990. Preliminary observations of lung injury produced by instillation
of HF in acidic and neutral buffer. Brainard JR, Kinkead SA, Kober
EM, Sebring RJ, Stavert DM. Los Alamos National Lab., NM. Supporting
Agency: Department of Energy, Washington, DC. Report No. NTIS/DE91009941.
Abstract:
The pathologic effects of exposure to combustion
products of polytetrafluoroethylene (9002-84-0) (PTFE)
were studied in rats. Fischer-344-rats received single 30 minute
exposures to concentrations from 0.005 to 5.025 milligrams per
liter aerosol products of PTFE heated to 595 degrees-C. The median
lethal concentration (LC50) was determined. Necropsies were performed
at 0, 2, 12, 24, and 36 hours post exposure or between 2 and 17
days. The LC50 for thermal degradation products of PTFE was 0.045
milligrams per liter. Conjunctival erythema
and serous occular and nasal discharge were seen in survivors
immediately after exposure. Lesions were
found in lungs of 84 percent of exposed rats. Focal hemorrhages,
edema, and fibrin deposition in the lungs were found. Focal interstitial
thickenings developed and increased. Alveolar macrophages became
more severe up to 96 hours. Thrombosis or embolism of pulmonary
capillaries and veins were found in 38 percent of exposed rats.
The degree of pathologic change increased as the dose increased
up to the LC50, but fluctuated above that. Disseminated intravascular
coagulation occurred in 53 percent of exposed rats and was positively
related to the amount of lung damage. Renal infarcts due to disseminated
intravascular coagulation were found but no other kidney lesions
were seen. The authors conclude that disseminated intravascular
coagulation appears to be a consequence of exposure to PTFE combustion
products.
Ref:
Pathologic Findings In Rats Following Inhalation Of Combustion
Products Of Polytetrafluoroethylene (PTFE) by Zook BC, Malek DE,
Kenney RA. Toxicology, Vol. 26, No. 1, pages 25-36, 1983.
Abstract:
An ultrastructural study was performed on the respiratory system
of budgerigars (including 6 controls)
which were acutely affected by inhalation of toxic fumes from
heated polytetrafluoroethylene (PTFE pyrolysis products) or had
survived for 24 h after a sublethal exposure. The controls were
exposed to fumes from heated plain Al (not coated with PTFE).
The microanatomy of lungs of the controls was described and compared
with that of lungs of birds exposed to PTFE pyrolysates. The
PTFE pyrolysates caused extensive, severe necrotizing and hemorrhagic
pneumonitis. These lesions were associated with amorphous
elongate conglomerates of particles which were similar to those
isolated on membrane filters from fumes generated from heated
PTFE. This supported the hypothesis that the toxic principle in
PTFE pyrolysates was related to generated particulates.
Ref:
Acute toxicosis of budgerigars (Melopsittacus undulatus) caused
by pyrolysis products from heated polytetrafluoroethylene: Microscopic
study by WELLS RE, SLOCOMBE RF. Astract: AM J VET RES; 43 (7).
1982. 1243-1248.
Abstract:
An incident where five cockatiels
(Nymphicus hollandicus) died within 30 minutes
following exposure from a frying pan coated with the plastic polytetrafluoroethylene
(900-284-0) (PTFE) that had accidentally overheated
is reported. Within an hour the owner developed
symptoms of polymer fume fever but recovered within 24
hours. A PTFE coated milk pan boiled dry for 15 minutes with the
cockatiels in a cage in the next room. The
birds were examined by a veterinary surgeon and were found to
be normal except for the lungs, which were
severely congested and edematous and were considered to be the
cause of death. It is concluded that parakeets are unusually
susceptable to the pyrolyses products of frying pans coated with
plastic polytetrafluoroethylene.
Ref.
Case of Polytetrafluoroethylene Poisoning in Cockatiels Accompanied
by Polymer Fume Fever in the Owner by Blandford TB, Hughes R,
Seamon PJ, Pattison M, Wilderspin
MP. Veterinary
Record, Vol. 96, pages 175-176, 6 references, 1975.
Abstract: A case of marked progression
of chronic obstructive pulmonary disease after several
episodes of occupational inhalation fever in a carding machine
operator was reported. The patient was a 45 year old male with
a history of exertional dyspnea who experienced recurrent episodes
of flu like symptoms beginning 2 weeks after starting work at
a synthetic textile plant. After approximately 9 months on the
job the patient was hospitalized with fever, chills, chest pain,
productive cough, and malaise that had not responded to antibiotic
treatment. A decreased white cell count
was seen along with evidence of moderately severe obstructive
disease. The patient returned to work after the acute symptoms
resolved; however, he experienced dyspnea
with mild exertion at this time. The flu
like illnesses continued to recur over the next 18 months
at which time the patient stopped working on the advice of his
physician. He was hospitalized 1 month later with chest
pain and diaphoresis. Severe obstruction with a significant
bronchodilator response was seen and he was placed on disability
leave. Polymer fume fever due to exposure
to polytetrafluoroethylene (9002-84-0) was suspected as the cause
of his illness. A subsequent examination of the patient's
workplace demonstrated that major renovations had been done since
his departure to improve chemical contamination and air quality;
however, potential for significant exposures to formaldehyde (50000)
were still evident. The authors conclude that polymer fume fever
may not always be a benign, self limiting disease and may result
in permanent airways damage. Long term follow up is recommended.
Ref: Progression of Chronic Obstructive
Pulmonary Disease after Multiple Episodes of an Occupational Inhalation
Fever by Kales SN, Christiani DC. Journal of Occupational Medicine,
Vol. 36, No. 1, Grant No. T15-OH-07096, pages 75-78, 10 references,
1994.
Abstract: Background: Certain fluorocarbon polymers can produce
a clinical syndrome called polymer fume
fever when the products of pyrolysis are inhaled. Summary:
A previously healthy 21-year-old white man presented with severe
chest tightness, difficulty in breathing, pyrexia, nausea, vomiting,
and a dry irritating cough. These symptoms occurred suddenly
while smoking a cigarette 2 hours after leaving his place of work,
where he is a plastics machinist. A chest roentgenogram revealed
a bilateral patchy alveolar air space filling
pattern involving the mid and lower lung fields.
The diagnosis of polymer
fume fever was established
on the basis of the symptom complex, the association with cigarette
smoking, and the occupational
exposure to micronized polytetrafluoroethylene.
Conclusions: A thorough occupational and smoking history is necessary
to recognize polymer fume disease, which may resemble influenza.
Ref: Acute noncardiogenic pulmonary edema
due to polymer fume fever. by SILVER MJ, YOUNG DK. CLEVELAND CLINIC
JOURNAL OF MEDICINE; 60 (6). 1993. 479-482.
Abstract: Polymer fume fever and other syndromes related to the
inhalation of fluoropolymer pyrolysis products were discussed
in this review. Polymer fume fever has primarily
been seen following the inhalation of thermal decomposition products
of materials such as polytetrafluoroethylene (9002-84-0) (PTFE),
fluorinated ethylene-propylene (61789002), and perfluoroalkoxyethylene
resins. The disease has been identified in plastics, chemical,
electronics, textile manufacturing, rubber stamp shop, print shop,
and aircraft repair shop workers as well as in several isolated
occupational and residential incidents. The pyrolysis and combustion
chemistry of PTFE and other fluoropolymers was discussed.
Animal studies on fluoropolymer inhalation toxicology and pathology
have demonstrated pulmonary hemorrhage,
edema, focal emphysema, and interstitial fibrosis and the
toxicity varied with the temperature of pyrolysis, the test animal,
length of study, and the presence or absence of particles. Early
clinical features in humans have included
flu like symptoms, chest discomfort, leukocytosis with a left
shift, an elevated erythrocyte sedimentation rate, and
inconsistent pulmonary function alterations. The disease
has generally tended to be acute and self limiting however late
sequelae such as pulmonary infiltrates and
persistent ventilatory impairment have been documented.
A crude dose response relationship for cases with severe
pulmonary damage has been suggested. The
use of urinary fluoride levels for biological monitoring of fluoride
exposure was discussed. Exposure standards established
by the OSHA were presented.
Ref: Polymer Fume Fever and Other Fluorocarbon
Pyrolysis-Related Syndromes. by Shusterman DJ. Occupational Medicine:
State of the Art Reviews, Vol. 8, No. 3, pages 519-531, 66 references,
1993.
Abstract: Perfluoroisobutene (PFIB) is
produced by the pyrolysis, and as a by-product during the manufacture,
of polytetrafluoroethylene. When inhaled it produces a
fulminating and sometimes fatal pulmonary oedema similar to that
of phosgene after a latent peroid of 6-8 h. As part of
a study to determine the retained dose and the factors that control
the amount retained, this study has investigated the retention
in rats of inhaled PFIB at concentrations of 10, 50 and 250 mugl/-1
in a flow-through system combining head-only exposure and plethysmography.
Uptake of PFIB was measured by gas chromatography during elevated
and reduced inspired volume and respiratory rate induced by exposure
to increased CO2 and injection of pentobartione, respectively.
The percentage of PFIB retained in the upper
airways and lungs was found to be 27.5, 28.1 and 23.7% of the
amount inspired at the three concentrations tested. The
rate of uptake (nmol min-1 kg-1) of PFIB was a power law of the
amount inha [astract truncated]
Ref: Retention of inhaled perfluoroisobutene
in the rat. by MAIDMENT MP, UPSHALL DG. J APPL TOXICOL; 12 (6).
1992. 393-400.
Abstract: Fluoropolymers, especially polytetrafluoroethylene
(PTFE), have good fire-resistance properties, but their application
is limited by concerns over the toxicity of their thermal decomposition
products. In experiments using a tube furnace system similar to
the DIN 53 436 method, the 30-minute (+ 14 days observation) LC50
im mass loss terms was found to be 2.9 mg l-1 (Standard Error
0.04) under non-flaming conditions, approximately ten times as
toxic as wood and most other materials.
Toxicity was due to upper respiratory tract and airway irritation,
and was consistent with the known effects of carbonyl fluoride
and hydrogen fluoride. When decomposed in the NBS cup furnace
test under non-flaming conditions, PTFE evolved extreme-toxicity
products with an LC50 of approximately 0.05 mg l-1 (mass loss),
approximately 1000 times as toxic as wood and most other materials.
Toxicity was due to deep lung irritation
and oedema. Investigations of the range of conditions under
which the [abstract truncated]
Ref: Recent developments in understanding
the toxicity of PTFE thermal decomposition products. by PURSER
DA. FIRE MATER; 16 (2). 1992. 67-75.
Abstract: The toxic effects of thermal
degradation products of polytetrafluoroethylene (9002-84-0) (PTFE)
and tetrafluoroethylene (116-14-3) / hexafluoropropylene (116-15-4)
copolymer (FEP) were studied in rats. Twenty two male Crl:CD-BR-rats
were exposed to FEP pyrolysis products in a National Bureau of
Standards (NBS) exposure chamber for 30 minutes; 4 rats were killed
1 to 4 hours after for microscopic examination of lung and nasal
sections and the remaining 18 were observed for 24 hours post
exposure. Four rats exposed to air only were used as controls.
Further experimentation involved exposure of 14 rats to PTFE pyrolysis
products in a low temperature decomposition exposure system for
4 hours; again, some rats were killed 1 to 4 hours thereafter
for microscopic evaluations and the remaining rats were observed
for 24 hours. Determinations
were made regarding the presence of gaseous hydrogen fluoride
and particle size. An intratracheal
instillation experiment was also conducted in which rats were
exposed to 0.05 milligrams aged PTFE particulate agglomerates
and their lungs were examined microscopically. In the NBS chamber,
the approximate lethal concentration (ALC) of particulate generated
was 0.3mg/m3 of FEP pyrolysis products; in the second chamber,
the ALC of fume evolved from PTFE was 0.1mg/m3. Fresh particles,
0.02 to 0.15 micrometers, were thought to be the toxic agents.
In the inhalation studies, exposed rats died from pulmonary congestion
and edema but only minimal respiratory epithelial damage was seen
in the nasal cavity or airways. Pulmonary
lesions were characterized by alveolar and interstitial edema
and intraalveolar hemorrhage due to damaged Type-I pneumocytes
and were associated with alveolar capillary neutrophilia.
The authors recommended investigation of the mode of action of
PTFE particulates on Type-I pneumocytes.
Ref: Pulmonary Response of Rats Exposed
to Polytetrafluoroethylene and Tetrafluoroethylene Hexafluoropropylene
Copolymer Fume and Isolated Particles. by Lee KP, Seidel WC. Inhalation
Toxicology, Vol. 3, No. 3, pages 237-264, 53 references, 1991.
• FORMATION OF ACUTE
PULMONARY TOXICANTS FOLLOWING THERMAL DEGRADATION OF PERFLUORINATED
POLYMERS EVIDENCE FOR A CRITICAL ATMOSPHERIC REACTION.
by WILLIAMS SJ, BAKER BB,
LEE K-P. FOOD CHEM TOXICOL; 25 (2). 1987. 177-186. No
abstract.
Abstract: The effects of flame retarded plastics on toxicity
were investigated in male albino Sprague-Dawley-rats. The substances
used in the investigation were: polytetrafluoroethylene
(9002-84-0) (PTFE), a copolymer of vinylidene fluoride
and hexafluoropropene (116-15-4) (VF), a copolymer of VF/HFP with
unidentified additives, and a polymer of VF/HFP and tetrafluoroethylene
(116-14-3). The fluoropolymer samples consisted of 16 millimeter
(mm) plugs with a 6mm hole in the middle, shaved until the desired
weight was obtained. When ambient system pressure was reached,
animals were dropped in an exposure chamber. Exposures were conducted
for 30 minutes and the rats were removed for observation. Animals
were necropsied for examination. Supplemental pyrolysis exposures
were conducted at the calculated maximum sublethal dose of each
fluoropolymer. The three VF/HFP substances were similar in that
complete degradation took place at 525 to 550 degrees-C. The comparable
temperature required for PTFE was 625 degrees. Rats
exposed to lethal doses of the pyrolysates showed lesions with
initial capillary damage leading to pulmonary edema, cell hypertrophy,
and desquamation. After 1 week post exposure, lungs returned
to normal. Exposures to doses of the pyrolysates at sublethal
values were similar but differed in that the degree of damage
was more restricted. The authors conclude that the three hydrogen
containing elastomers produced a less toxic pyrolysate than PTFE.
Identification of the compounds responsible for the pulmonary
damage is presently being carried out.
Ref: Toxicity Associated With Flame-Retarded
Plastics . by Carter VL Jr. Bafus DA, Warrington HP, Harris ES.
Physiological and Toxicological Aspects of Combustion Products:
International Symposium, National Academy of Sciences, Washington,
D.C., pages 96-104, 4 references, 1976.
Abstract: The relative toxicities of thermal degradation products
from four fluoropolymers were determined in rats. The polymers
were polytetrafluoroethylene (9002-84-0)
(PTFE), copolymers of vinylidene fluoride (75387) and hexafluoropropene
(116-15-4) with and without additives (VF2-A and VF2/HFP), and
the terpolymers of PTFE, VF2, and HFP (VF2/HFP/TFE). Male Sprague-Dawley-rats
were exposed for 30 minutes to the pyrolysis products of VF2/HFP,
VF2/HFP-A, and VF2/HFP/TFE at 550 and 800 degrees-C, and to the
pyrolysis products of PTFE at 625 and 800 degrees-C. Survivors
were killed at 1, 2, 4, 8, 16, and 32 days postexposure and examined
for pathological changes in organs and tissues. Hydrolyzable fluoride
concentrations were determined for pyrolysis products of all polymers
at both temperatures. At 550 degrees-C, the median lethal dose
ranged from 1.06 grams (g) VF2/HFP-A to 2.36g VF2/HFP. The
median lethal dose of PTFE at 625 degrees-C was 0.5g. At 800 degrees-C,
the median lethal dose ranged from 0.38g PTFE (for a 5 minute
exposure) to 0.59g VF2/HFP (for a 30 minute exposure).
Initial pathological response to pyrolysates included capillary
damage leading to pulmonary edema, and alveolar hypertrophy and
desquamation. For survivors, edema was resolved and a proliferative
phase began after 48 hours. Lungs returned to normal after 1 week.
No other organ system was involved. No relationship was found
between hydrolyzable fluoride concentrations of pyrolysates and
toxicities. The authors conclude that the
pyrolysis products of PTFE are more toxic than those of polymers
containing VF2 and HFP.
Ref: The Acute Inhalation Toxicity
in Rats from the Pyrolysis Products of Four Fluoropolymers. by
Carter VL Jr, Bafus DA, Warrington HP, Harris ES. Toxicology and
Applied Pharmacology, Vol. 30, No. 3, pages 369-376, 9 references,
1974.
Abstract: Studies
by the du Pont Company investigating the toxicity
of pyrolysis products of two tetrafluoroethylene (TFE) resins,
Teflon-1 and Teflon-6 (9002-84-0)
are reviewed. TFE resins are physiologically inert, but when subjected
to temperatures of 300 degrees-C and above, toxic effects in rats
are observed. In addition
to causing death of experimental animals, toxic symptoms include
pulmonary congestion and edema, bronchitis, bronchopneumonia,
chronic pneumonia, and emphysema. As temperature
increases above 300 degrees-C, the rate of thermal decomposition
increases and the pyrolysis products change.
Small quantities of carbon dioxide and fluorides that do not appear
to be important toxicologically are evolved when TFE resins are
exposed to temperatures from 300 to 350 degrees-C. However, a
fine particulate material evolving during pyrolysis is toxic to
rats. The observed toxicity of pyrolysis products heated to 380
degrees-C and above may be due chiefly to octafluoroisobutylene
[also known as Perfluoroisobutylene]
(382-21-8). The relative toxicity
of pyrolysis products of Teflon-1 and Teflon-6 corresponds to
the amount of matter evolved. Filtration of the pyrolysis stream
significantly reduces the toxicity of the products evolved at
temperatures of 325 and 350 degrees-C. New
manufacturing techniques for TFE resins have resulted in products
with improved thermal stability and corresponding lower toxicity
of pyrolysis products. The authors conclude that experimental
work is still needed to allow exact characterization of the toxic
pyrolysis products of TFE resins.
Ref: The Toxicity of the Pyrolysis Products
of "Teflon" TFE-Fluorocarbon Resins. Clayton JW Jr,
Hood DB, Raynsford GE. Haskell Laboratory for Toxicology and Industrial
Medicine, E. I. du Pont de Nemours and Co., Inc., Wilmington,
Delaware, Presented at the American Industrial Hygiene Association
Annual Meeting, 9 pages, 1959.
Abstract: Teflon (9002-84-0) vapor toxicity under various thermal
conditions is reviewed. It is noted that under normal usage conditions
Teflon is among the most inert, nontoxic, and nonflammable substances
tested. The primary uses of Teflon are described. Its nontoxic
nature has been proven by animals tests. At 200 degrees-C or higher
Teflon decomposes to release toxic fumes which cause polymer fume
fever in humans. The incidence of this reaction among Teflon workers
is discussed and the symptoms described. It is noted that the
effects last for 1 to 2 hours but rarely longer, and recovery
is complete within 24 to 36 hours. The rate of decomposition of
Teflon resins at various temperatures ranging from 200 to 500
degrees is described and the products of pyrolysis at these temperatures
are specified. The major component of pyrolysis
at 400 degrees is the monomer tetrafluoroethylene (116-14-3)
a relatively innocuous gas which comprises at least 95 to 97 percent
of the gaseous output and hexafluoropropylene
(116-15-4) (2 to 3 percent). Trace
amounts of hydrogen fluoride (7664393) have also been detected
especially in the presence of moisture during Teflon pyrolysis,
as well as perfluoroisobutylene (382-21-8).
The toxicity of the latter two compounds
in animals is discussed. The toxic response in animals
is reviewed in terms of human maximal allowable concentrations.
Other features of the pyrolytic decomposition
of Teflon such as weight loss at various temperatures is tabulated.
Control measures for storage of Teflon and other fluorocarbon
plastics, fabrication, installation and maintenance of components
utilizing Teflon and disposal of scrap Teflon are discussed as
well as personal hygiene, ventilation during heating of Teflon,
use of emergency personal protective equipment, and maintenance
of adequate medical records. Ref:
Environmental And Occupational Health Information Letter Number
13A. Toxicity Of Products Of Thermal Decomposition Of Teflon.
Anonymous. Office of the Surgeon, Air Material Command, Wright-Patterson
Air Force Base, Ohio, 7 pages, 9 references, 1958.