|
Return to Phototoxic Pesticides
Abstracts
KEYWORDS:
photocarcinogenic, photocarcinogenicity
photoclastogenic
photocytotoxic
photogenotoxic
photohemolytic
photomutagenic, photomutagenicity
phototoxic, phototoxicity
| |
|
|
|
| Click
here |
Click
here |
Click
here |
Click
here |
Bay
Y3118
Ciprofloxacin
Clinafloxacin
Enoxacin
Fleroxacin |
Gemifloxacin
Grepafloxacin
Levofloxacin
Lomefloxacin |
Moxifloxacin
Norfloxacin
Ofloxacin
Pefloxacin
Sitafloxacin |
Sparfloxacin
Temafloxacin
Tosufloxacin
Trovafloxacin |
| Name: |
Sparfloxacin |
| CAS No. |
110871-86-8
and (111542-93-9) |
| Formula: |
C19-H22-F2-N4-O3
|
| Structure: |
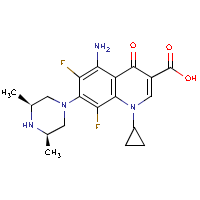 |
| Other Names: |
AT 4140
AT-4140
BRN 3658018
CI 978
CI-978
CP 103826
DRG-0143
Esparfloxacino [INN-Spanish]
PD-131501
Sparfloxacin
Sparfloxacine [INN-French]
Sparfloxacinum [INN-Latin]
Zagam |
| Class |
Fluoroquinolone |
Effects
(some, not all) |
•
Photogenotoxic
• Photoclastogenic
• Phototoxic
• Teratogenic effects
• Can prolong the QT interval to cause lethal ventricular
arrhythmias - withdrawn in most countries. |
| Use: |
Antibacterial
Drug / Therapeutic Agent
Withdrawn
in most countries. |
Sparfloxacin
Abstracts:
AIM:
To compare two methods of measuring DNA damage induced by photogenotoxicity
of fluoroquinolones (FQ).
METHODS: Lomefloxacin (LFLX), sparfloxacin
(SPFX), ciprofloxacin (CPFX), and levofloxacin (LELX) were
tested by comet assay and photodynamic DNA strand breaking activity
under the different conditions of UVA irradiation.
RESULTS: In comet assay, photogenotoxicity
was evident at SPFX 1 mg/L, LFLX 5 mg/L, and CPFX 5 mg/L,
and LELX 10 mg/L. In photodynamic DNA strand-breaking activity,
SPFX and LFLX induced the conversion
of the supercoiled form into the nicked relaxed form at 10-50
micromol/L, while CPFX at 25 micromol/L and LELX at 50 micromol/L.
CONCLUSION: There were good correlations between the two methods
to detect DNA damage induced by phototoxicity of fluoroquinolones.
Photodynamic DNA strand breaking activity was a good method to
detect DNA damage induced by photogenotoxicity
of fluoroquinolones as well as comet assay.
Ref: Compare two methods of measuring DNA
damage induced by photogenotoxicity of fluoroquinolones. By Zhang
T, Li JL, Xin J, Ma XC, Tu ZH. Acta Pharmacol Sin. 2004 Feb;25(2):171-5.
... In fetuses,
decreased body weight, increased incidence of ventricular septal
defect, decreased incidence of 14th ribs, and delayed ossification
were found in the 300 mg/kg dose group...
Ref: [Reproductive and developmental toxicity
studies of sparfloxacin (2) -- teratogenicity study in rats];
by H Funabashi et al. Yakuri To Chiryo 1991 Apr;19(4):69-86.
Sparfloxacin interfered
slightly with limb bud growth at 30 mg/L; none of the other
fluoroquinolones impaired development at this concentration.
Ref: Effects of fluoroquinolones in a mouse
limb bud culture system using regular and magnesium-deficient
medium; by R Stahlmann et al. Teratology 1997 Jan;55(1):61-2.
... Teratogenic
effects have been observed in animals treated with the
oldest quinolones (flumequine, nalidixic acid and pipemidic acid)
and also with sparfloxacin, a fluoroquinolone...
Ref: Quinolones and pregnancy: worrying
animal findings, few clinical data. Prescrire Int 1999 Feb;8(39):29-31.
The phototoxic potential
of eight fluoroquinolones (norfloxacin, ofloxacin, enoxacin, ciprofloxacin,
lomefloxacin, tosufloxacin, sparfloxacin
and gatifloxacin) was evaluated by using three in vitro methods
of cytotoxicity against mammalian cells, erythrocyte lysis and
DNA strand breakage. All fluoroquinolones
tested with the exception of gatifloxacin, an 8-methoxy quinolone,
showed DNA strand breaking activities under UV-A irradiation.
Their cytotoxicity against HeLa cells was also enhanced by UV-A
irradiation. In particular, the phototoxic
potential of sparfloxacin, enoxacin and lomefloxacin was high
in both methods. Ofloxacin is very photocytotoxic against
HeLa cells, while it has low potential to cause DNA strand breakage.
Norfloxacin, ciprofloxacin and enoxacin were very photohemolytic,
but sparfloxacin was not, indicating that the in vivo phototoxic
potencies of fluoroquinolones might not be predictable by the
photohemolysis study. Gatifloxacin, a non-phototoxic quinolone,
showed no phototoxic potential in any of these three in vitro
tests. These results suggest that determination of DNA strand
breaking activity, combined with cytotoxicity against mammalian
cells, is available to predict the phototoxic potential of fluoroquinolones
without laboratory animals.
Ref: In vitro method for prediction of the
phototoxic potentials of fluoroquinolones; by T. Yamamoto et al.
Toxicology in Vitro - Volume 15, Issue 6 , December 2001, Pages
721-727.
The photochemical clastogenic
potential of 12 quinolone antibacterial agents with or without
light irradiation was assessed by an in vitro chromosomal aberration
test using cultured CHL cells. Exposure to all test compounds,
except for DK-507k, increased the incidence of cells with structural
aberrations excluding gap (TA) following light irradiation. Test
compounds used in the present study under light irradiation were
divided into three groups based on their ED50 values, doses inducing
chromosomal aberrations in 50% of cells. The
first group with ED50 values below 30 g/ml includes sparfloxacin
(SPFX), clinafloxacin (CLFX), gemifloxacin (GMFX), lomefloxacin
(LFLX), sitafloxacin (STFX), grepafloxacin (GPFX) and fleroxacin
(FLRX); the second group with ED50 values of 100 g/ml, enoxacin
(ENX) and levofloxacin (LVFX); the third group with little
or no potency, moxifloxacin (MFLX), trovafloxacin (TVFX) and DK-507k.
The photochemical clastogenicity of these
compounds correlates well with their reported in vivo phototoxic
potentials. In the chemical structure
and clastogenicity relationships, substitution of a methoxy group
at the C-8 position in the quinolone nucleus was confirmed to
reduce not only photochemical clastogenicity, but also the clastogenic
potential of quinolone antibacterial agents.
Ref: In vitro photochemical clastogenicity
of quinolone antibacterial agents studied by a chromosomal aberration
test with light irradiation. By Satoru Itoh et al. Mutation Research/Genetic
Toxicology and Environmental Mutagenesis Volume 517, Issues 1-2
, 27 May 2002, Pages 113-121.
Excerpts: Since noncardiovascular
drug-induced prolongation of the QT interval is often associated
with the onset of torsades de pointes resulting in life-threatening
ventricular arrhythmias (De Ponti et al., 2001; Haverkamp et al.,
2000 and Tamargo, 2000), worldwide regulatory authorities have
raised a heightened awareness on the submission of data surrounding
the ventricular repolarization process. Moreover, general nonclinical
testing strategy for delayed ventricular repolarization by human
pharmaceuticals is being discussed in draft stage guideline ICH
S7B for safety pharmacology studies (The ICH Steering Committee,
2002).
In the case of fluoroquinolone antibacterial agents, it has been
reported that sparfloxacin and grepafloxacin
can prolong the QT interval to cause lethal ventricular arrhythmias
(Bertino and Fish, 2000; Demolis et al., 1996; Dupont et
al., 1996 and Owens, 2001), which were withdrawn
in most countries. Recently, gatifloxacin and moxifloxacin
were developed as third generation of fluoroquinolones (Ball,
2000). However, in vitro studies have indicated
that gatifloxacin and moxifloxacin markedly prolonged the action
potential duration of the isolated guinea pig ventricular myocardium
and canine Purkinje fibers (Gintant et al., 2001; Hagiwara
et al., 2001 and Patmore et al., 2000). Also, gatifloxacin
and moxifloxacin inhibited the human cardiac repolarizing K+ current
(Anderson et al., 2001; Bischoff et al., 2000 and Kang et al.,
2001). Clinical studies on the safety pharmacology of gatifloxacin
and moxifloxacin indicated that these fluoroquinolones may induce
QT prolongation and ventricular arrhythmias (Bertino et
al., 2002; Démolis et al., 2000; Iannini and Circiumaru,
2001; Noel et al., 2003; Siepmann and Kirch, 2001 and Von Keutz
and Schlüter, 1999).
Ref: In vivo experimental approach for the
risk assessment of fluoroquinolone antibacterial agents-induced
long QT syndrome; by Katsuyoshi Chiba et al. European Journal
of Pharmacology Volume 486, Issue 2 , 20 February 2004, Pages
189-200.
The new fluoroquinolones (clinafloxacin, gatifloxacin, gemifloxacin,
grepafloxacin, levofloxacin, moxifloxacin, sitafloxacin, sparfloxacin
and trovafloxacin) offer excellent activity against Gram-negative
bacilli and improved Gram-positive activity (e.g. against Streptococcus
pneumoniae and Staphylococcus aureus) over ciprofloxacin... Several
of these agents have either been withdrawn from the market, had
their use severely restricted because of adverse effects (clinafloxacin
because of phototoxicity and hypoglycaemia; grepafloxacin
because of prolongation of the QTc and resultant torsades de pointes;
sparfloxacin because of phototoxicity;
and trovafloxacin because of hepatotoxicity), or were discontinued
during developmental phases. The remaining fluoroquinolones such
as gatifloxacin, gemifloxacin, levofloxacin and moxifloxacin have
adverse effect profiles similar to ciprofloxacin. Extensive post-marketing
safety surveillance data (as are available with ciprofloxacin
and levofloxacin) are required for all new fluoroquinolones before
safety can be definitively established. Drug interactions are
limited; however, all fluoroquinolones interact with metal ion
containing drugs (eg. antacids)..
Ref: A critical review of the fluoroquinolones:
focus on respiratory infections; by GG Zhanel et al. Drugs. 2002;62(1):13-59.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11790155
Significant safety issues that have arisen with fluoroquinolones
include phototoxicity, cardiotoxicity, tendinitis, CNS effects
and drug interactions. Ciprofloxacin is well tolerated; the incidence
of adverse events is low and serious adverse events are rare.
Levofloxacin has a reduced CNS adverse event rate compared with
ofloxacin. Sparfloxacin has significant
phototoxicity and potential cardiac toxicity. Grepafloxacin
has significantly increased adverse event rates, particularly
gastrointestinal intolerance. Taste perversion and nausea are
common. Trovafloxacin has an increased potential
for CNS adverse reactions, notably dizziness. Post-marketing surveillance
data indicate the possibility of serious hepatic reactions and
pancreatitis. Interactions between fluoroquinolones and
drugs metabolised by the hepatic cytochrome P450 system affect
the clearance of theophylline and caffeine. Quinolone absorption
is significantly reduced by co-administration of antacids. Hospitalised
patients are likely to be receiving multiple-drug therapy, but
drug interactions are avoidable. The interactions of specific
fluoroquinolones should be checked prior to prescription.
Ref: Safety of the new fluoroquinolones
compared with ciprofloxacin. By P Ball. J Chemother. 2000 Jan;12
Suppl 1:8-11.
| Name: |
Temafloxacin |
| CAS No. |
108319-06-8
|
| Formula: |
C21-H18-F3-N3-O3
|
| Structure: |
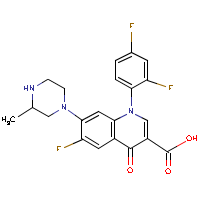 |
| Other Names: |
Omniflox
A 62254
BRN 4301726
CCRIS 6303
T 1258 |
| Class |
Fluoroquinolone |
Effects
(some, not all) |
•
Embryolethal (animal study)
This drug was on the market for 3 months
in 1992 when
Abbot Laboratories "voluntarily" recalled it. FDA
cited the following "severe adverse effects" in
its announcement of the recall:
• Hemolytic anemia (destruction of red blood cells)
and other blood cell abnormalities (severe
hemolytic-uremic syndrome)
• Kidney dysfunction
• Liver dysfunction
• Allergic
reactions, some of which have caused life-threatening respiratory
distress
|
| Use: |
Anti-infective drug
•
Withdrawn from worldwide markets due to adverse effects.
|
Temafloxacin Abstracts:
....... The Food and Drug Administration
today announced that Abbott Laboratories of Abbott Park, Ill.,
is voluntarily recalling the broad-spectrum anti-infective drug
Omniflox (temafloxacin) tablets, and will halt all further distribution
of the drug.
....... This action is being taken
because of severe adverse events associated
with the use of the drug that have been reported to the company
and to FDA in the first three months of marketing.
....... Temafloxacin was approved
in late January 1992 and marketed in mid-February. Since that
time there have been approximately 50 reports of serious adverse
reactions, including three deaths. There were several cases of
severe low blood sugar, especially
in very elderly patients with decreased kidney function. Among
the severe reactions there were a number
of cases of an unusual complex of adverse reactions consisting
of hemolytic anemia (destruction of red blood cells) and other
blood cell abnormalities. Also observed were patients with
kidney dysfunction, about half of
which required renal dialysis. Other patients suffered liver dysfunction.
....... There has also been a substantial
number of reports of allergic reactions,
some of which have caused life-threatening respiratory distress.
....... Temafloxacin is one of a
newer class of synthetic oral fluoroquinolones -- broad-spectrum
antibiotics -- that are used to treat a variety of infections
including lower respiratory tract infections, skin and skin structure
infections, infection of the prostate and urinary tract infections.
Similar antibiotics of its class have not been reported to be
associated with comparable numbers of serious adverse reactions.
....... Consumers who have the medication
are advised to consult their physician and return any unused portions
of the product to the place of purchase.
....... FDA is one of the eight Public
Health Service agencies within HHS.
Ref: June 5, 1992: Press Release from the
US Food and Drug Administration.
http://www.fda.gov/bbs/topics/NEWS/NEW00279.html
Clinical trials in patients with community- and hospital-acquired
infections have established that the clinical effectiveness and
safety of fluoroquinolones are similar to -lactam and macrolide
agents. The most common drug-related adverse
effects (AEs) with fluoroquinolone therapy involve the
gastrointestinal troct and central nervous system and are usually
transient and mild to moderate in severity. However, serious toxic
reactions have led to the limited and restrictive use of trovafloxacin
in the United States and the withdrawal
of temafloxacin and grepafloxacin from worldwide markets.
In addition, postmarketing spontaneous AE reports have imposed
updates in the precautions and warning sections of product package
inserts of selected fluoroquinolones. This
article reviews the AEs associated with the fluoroquinolones and
compares the safety profiles of ciprofloxacin, levofloxacin, gatifloxacin,
and moxifloxacin.
Ref: Safety and tolerability of fluoroquinolones;
by Kelly A. Sprandel PharmD and Keith A. Rocivold PharmD, FCP,
FCCP. Clinical Cornerstone Volume 5, Supplement 3 , 2003, Pages
S29-S36.
The potential developmental toxicity of fleroxacin was studied
(Phase I) and its pharmacokinetics was compared to ciprofloxacin,
temafloxacin, and norfloxacin (Phase II) in the cynomolgus macaque
(Macaca fascicularis). Phase I studies involved oral administration
of fleroxacin (35 and 70 mg/kg-day) during Gestational Days (GD)
20-34 or 35-49 (N = 10/group); controls received vehicle only.
Increased maternal toxicity (weight loss, anorexia, emesis) and
embryolethality (4/10, 40%; GD 20-34) were observed at 70 mg/kg-day.
Urinary excretion of estrogen conjugates was reduced for females
with nonviable pregnancies during both treatment periods (GD 20-34
and 35-49), although steroid hormone levels in serum remained
unchanged during treatment; no malformations or growth retardation
were observed at gross examination. For Phase II studies, the
pharmacokinetics of fleroxacin (70 mg/kg), ciprofloxacin
(100 mg/kg), temafloxacin (100
mg/kg), and norfloxacin (150 mg/kg) were studied during a 3-day
oral treatment regimen in the nonpregnant (N = 12; 3/quinolone)
and pregnant (N = 3; fleroxacin only) macaque. Serial blood samples
were collected on the first and third days of treatment in all
animals; for pregnant females, the conceptus was removed on GD
31 for analysis of fleroxacin levels. Marked differences between
the quinolones were noted in the AUC0-24 hr for nonpregnant females.
Based on AUC0-24 hr on the first day of treatment, the rank order
was fleroxacin > temafloxacin > ciprofloxacin
> norfloxacin. On the third day of treatment, the rank order
for exposure was temafloxacin> fleroxacin > ciprofloxacin
> norfloxacin. Overall, results indicated (1) no marked differences
in pharmacokinetic parameters in pregnant versus nonpregnant females,
(2) fleroxacin levels in embryonic tissues were similar to maternal
plasma levels, and (3) there was a correlation between exposure
and embryolethal doses for all fluoroquinolones which resulted
in embryolethality except norfloxacin.
Ref: Developmental Toxicity of Fleroxacin
and Comparative Pharmacokinetics of Four Fluoroquinolones in the
Cynomolgus Macaque (Macaca fascicularis)
Hummler H., Richter W. F. and Hendrickx A. G. Toxicology and Applied
Pharmacology Volume 122, Issue 1 , September 1993, Pages 34-45.
Grepafloxacin
Abstracts:
Fluoroquinolone development from 1985 to the present was reviewed.
Severe drug adverse events were noted for enoxacin, pefloxacin
and fleroxacin, which were phototoxic. Temafloxacin
was associated with severe hemolytic-uremic syndrome, lomefloxacin
caused phototoxicity and central nervous system (CNS) effects,
and sparfloxacin was associated with phototoxicity and
QTc prolongation. Tosufloxacin caused severe thrombocytopenia
and nephritis, and hepatotoxicity was reported for trovafloxacin.
Grepafloxacin was withdrawn due to cardiovascular
effects, and clinafloxacin was associated with phototoxicity
and hypoglycaemia. The structure of the quinolones directly relates
to both their activity and side-effect profiles. The relationship
among specific substituents attached to the quinolone nucleus
are clarified. The incidence of specific adverse events associated
with individual fluoroquinolones was reviewed in a five-year post-marketing
surveillance (PMS) study in Japan, in which a total adverse drug
reaction (ADR) rate of 1.3% was found for levofloxacin, compared
to total ADR rates of 3.3% for pazufloxacin, 3.6% for tosufloxacin,
4.5% for gatifloxacin and 5.4% for balofloxacin. Gastrointestinal
effects were the most common adverse events for all fluoroquinolones.
Levofloxacin had the lowest rate of CNS effects and skin adverse
events among the agents listed.
Ref: History of quinolones and their side
effects by E Rubinstein. Chemotherapy. 2001;47 Suppl 3:3-8; discussion
44-8.
| Name: |
Tosufloxacin |
| CAS No. |
100490-36-6
|
| Formula: |
C19-H15-F3-N4-O3
|
| Structure: |
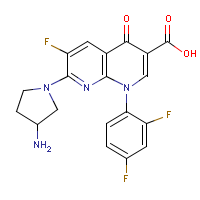 |
| Other Names: |
A 61827
A 67107
BRN 4913117
CCRIS 6304 |
| Class |
Fluoroquinolone |
Effects
(some, not all) |
•
Photogenotoxic
• Phototoxic
• Thrombocytopenia
• Nephritis |
| Use: |
|
Tosufloxacin
Absracts:
The phototoxic
potential of eight fluoroquinolones (norfloxacin, ofloxacin, enoxacin,
ciprofloxacin, lomefloxacin, tosufloxacin,
sparfloxacin and gatifloxacin) was evaluated by using three in
vitro methods of cytotoxicity against mammalian cells, erythrocyte
lysis and DNA strand breakage. All fluoroquinolones
tested with the exception of gatifloxacin, an 8-methoxy quinolone,
showed DNA strand breaking activities under UV-A irradiation.
Their cytotoxicity against HeLa cells was also enhanced by UV-A
irradiation. In particular, the phototoxic potential of
sparfloxacin, enoxacin and lomefloxacin was high in both methods.
Ofloxacin is very photocytotoxic against HeLa cells, while it
has low potential to cause DNA strand breakage. Norfloxacin, ciprofloxacin
and enoxacin were very photohemolytic, but sparfloxacin was not,
indicating that the in vivo phototoxic potencies of fluoroquinolones
might not be predictable by the photohemolysis study. Gatifloxacin,
a non-phototoxic quinolone, showed no phototoxic potential in
any of these three in vitro tests. These results suggest that
determination of DNA strand breaking activity, combined with cytotoxicity
against mammalian cells, is available to predict the phototoxic
potential of fluoroquinolones without laboratory animals.
Ref:
In vitro method for prediction of the phototoxic potentials of
fluoroquinolones; by T. Yamamoto et al. Toxicology in Vitro -
Volume 15, Issue 6 , December 2001, Pages 721-727.
Fluoroquinolone development from 1985 to the present was reviewed.
Severe drug adverse events were noted for enoxacin, pefloxacin
and fleroxacin, which were phototoxic. Temafloxacin
was associated with severe hemolytic-uremic syndrome, lomefloxacin
caused phototoxicity and central nervous system (CNS) effects,
and sparfloxacin was associated with phototoxicity and
QTc prolongation. Tosufloxacin caused severe
thrombocytopenia and nephritis, and hepatotoxicity was
reported for trovafloxacin. Grepafloxacin
was withdrawn due to cardiovascular effects, and clinafloxacin
was associated with phototoxicity and hypoglycaemia. The structure
of the quinolones directly relates to both their activity and
side-effect profiles. The relationship among specific substituents
attached to the quinolone nucleus are clarified. The incidence
of specific adverse events associated with individual fluoroquinolones
was reviewed in a five-year post-marketing surveillance (PMS)
study in Japan, in which a total adverse drug reaction (ADR) rate
of 1.3% was found for levofloxacin, compared to total ADR rates
of 3.3% for pazufloxacin, 3.6% for tosufloxacin, 4.5% for gatifloxacin
and 5.4% for balofloxacin. Gastrointestinal effects were the most
common adverse events for all fluoroquinolones. Levofloxacin had
the lowest rate of CNS effects and skin adverse events among the
agents listed.
Ref: History of quinolones and their side
effects by E Rubinstein. Chemotherapy. 2001;47 Suppl 3:3-8; discussion
44-8.
| Name: |
Trovafloxacin
|
| CAS No. |
147059-72-1
(and 146836-84-2) |
| Formula: |
C20-H15-F3-N4-O3
|
| Structure: |
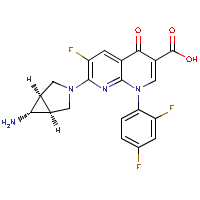 |
| Other Names: |
Trovan
|
| Class |
Fluoroquinolone |
Effects
(some, not all) |
•
Hepatotoxicity
• Diminished
healing during the early stages of fracture repair; may compromise
fracture healing in humans.
• Potential
for CNS adverse reactions, notably dizziness.
|
| Use: |
•
Limited and restrictive use in the United States
|
Trovafloxacin
Abstracts:
... Trovan (trovafloxacin/alatrofloxacin) is an example of a
drug with significant limitations of use because of the potential
for serious liver injury. The antimicrobial therapy treats
a variety of infections, from mild to life threatening. Though
no cases of liver failure, liver transplant, or death were reported
in the 7,000 patients who took part in premarketing clinical trials
for Trovan, the FDA began receiving reports
of liver failure after Pfizer began marketing the drug in 1998.
As a result, the FDA and Pfizer agreed to restrictions, which
include limiting distribution of Trovan to inpatient facilities
(hospitals, nursing homes) so doctors can closely monitor patients
taking the drug. Trovan use was also limited to the treatment
of patients with serious, life- or limb-threatening infections...
Ref: Serious Liver Injury: Leading Reason
for Drug Removals, Restrictions. By Michelle Meadows. FDA Consumer
magazine May-June 2001.
http://www.fda.gov/fdac/features/2001/301_liver.html
Excerpts: A group of Nigerian families has taken legal action
against the pharmaceutical company, Pfizer. The
lawsuit, which was filed in a US court on Aug 29, claims that
the families' children were entered into a trial of Pfizer's Trovan
(trovafloxacin) for bacterial meningitis in 1996 without informed
consent. The families accuse the US-based drug company
of violating “international law, federal regulations and
medical ethics, in its zeal to carry out its test”.
Pfizer gave the children a “new, untested and unproven drug
without first obtaining their informed consent, or explaining
to the children or their parents that the proposed treatment was
experimental and that they were free to refuse it and instead
choose the safe, effective treatment for bacterial meningitis
offered at the same site, free of charge, by a charitable medical
group”, according to the lawsuit... Pfizer said: “the
fatality rates in the Kano study, approximately 6% for both Trovan
and ceftriaxone, were lower than published results for other forms
of treatment in this epidemic.” But, the suit alleges that
the deaths and injuries among controls were the result of a lower
than recommended dose of ceftriaxone... The Trovan trial supports
the veracity of the international concerns that developing countries
will merely be used as test areas for medical treatments designed
for developed countries, adds Charles Weijer (Dalhousie University,
Halifax Nova Scotia, Canada). ... Weijer called on the international
community to guard against the exploitation of developing countries,
and ensure that all research participants receive equal protection.
“The pharmaceutical industry has, as illustrated in this
case, demonstrated its willingness to exploit the vulnerable.”
Ref: Drug company sued over research trial
in Nigeria by Khabir Ahmad. The Lancet - Volume 358, Issue 9284
, 8 September 2001, Page 815.
Clinical trials in patients with community- and hospital-acquired
infections have established that the clinical effectiveness and
safety of fluoroquinolones are similar to -lactam and macrolide
agents. The most common drug-related adverse
effects (AEs) with fluoroquinolone therapy involve the
gastrointestinal troct and central nervous system and are usually
transient and mild to moderate in severity. However, serious toxic
reactions have led to the limited and restrictive
use of trovafloxacin in the United States and the withdrawal
of temafloxacin and grepafloxacin from worldwide markets.
In addition, postmarketing spontaneous AE reports have imposed
updates in the precautions and warning sections of product package
inserts of selected fluoroquinolones. This
article reviews the AEs associated with the fluoroquinolones and
compares the safety profiles of ciprofloxacin, levofloxacin, gatifloxacin,
and moxifloxacin.
Ref: Safety and tolerability of fluoroquinolones;
by Kelly A. Sprandel PharmD and Keith A. Rocivold PharmD, FCP,
FCCP. Clinical Cornerstone Volume 5, Supplement 3 , 2003, Pages
S29-S36.
Idiosyncratic drug toxicity, defined as toxicity that is dose
independent, host dependent, and usually cannot be predicted during
preclinical or early phases of clinical trials, is a particularly
confounding complication of drug development. An understanding
of the mechanisms that lead to idiosyncratic liver toxicity would
be extremely beneficial for the development of new compounds.
We used microarray analysis on isolated human hepatocytes to understand
the mechanisms underlying the idiosyncratic toxicity induced by
trovafloxacin. Our results clearly distinguish trovafloxacin from
other marketed quinolone agents and identify unique gene changes
induced by trovafloxacin that are involved in mitochondrial damage,
RNA processing, transcription, and inflammation that may suggest
a mechanism for the hepatotoxicity induced
by this agent. In conclusion, this work establishes the basis
for future microarray analysis of new compounds to determine the
presence of these expression changes and their usefulness in predicting
idiosyncratic hepatotoxicity. Supplementary material for this
article can be found on the HEPATOLOGY website (http://interscience.
Wiley.com/jpages/0270-9139/suppmat/index.htnd).
Ref: Microarray analysis in human
hepatocytes suggests a mechanism for hepatotoxicity induced by
trovafloxacin; by MJ Liguori et al. Hepatology. 2005 Jan;41(1):177-86.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15619227
The new fluoroquinolones (clinafloxacin, gatifloxacin, gemifloxacin,
grepafloxacin, levofloxacin, moxifloxacin, sitafloxacin, sparfloxacin
and trovafloxacin) offer excellent activity against Gram-negative
bacilli and improved Gram-positive activity (e.g. against Streptococcus
pneumoniae and Staphylococcus aureus) over ciprofloxacin... Several
of these agents have either been withdrawn from the market, had
their use severely restricted because of adverse effects (clinafloxacin
because of phototoxicity and hypoglycaemia; grepafloxacin
because of prolongation of the QTc and resultant
torsades de pointes;
sparfloxacin because of phototoxicity; and trovafloxacin
because of hepatotoxicity), or were discontinued during
developmental phases. The remaining fluoroquinolones such as gatifloxacin,
gemifloxacin, levofloxacin and moxifloxacin have adverse effect
profiles similar to ciprofloxacin. Extensive post-marketing safety
surveillance data (as are available with ciprofloxacin and levofloxacin)
are required for all new fluoroquinolones before safety can be
definitively established. Drug interactions are limited; however,
all fluoroquinolones interact with metal ion containing drugs
(eg. antacids)...
Ref: A critical review of the fluoroquinolones:
focus on respiratory infections; by GG Zhanel et al. Drugs. 2002;62(1):13-59.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11790155
We previously have shown that experimental fractures exposed
to ciprofloxacin have diminished fracture healing. The purpose
of this study was to assess the effect of levofloxacin and trovafloxacin
on experimental fracture healing to test the hypothesis that diminished
fracture healing is a quinolone class effect. Sixty-one male Wistar
rats were divided into three groups, which received 25 mg/kg of
levofloxacin twice daily for 3 weeks, 35 mg/kg of trovafloxacin
twice daily for 3 weeks, or no treatment, beginning 7 days after
production of closed, nondisplaced, bilateral femoral fractures.
The mean peak serum concentrations of levofloxacin and trovafloxacin
drawn 30 minutes after administration were 6.9 and 7.0 microg/mL,
respectively. Radiographic, histologic, and biomechanical studies
were used to evaluate fracture healing. Torsional strength testing
of fracture callus exposed to levofloxacin and trovafloxacin revealed
a decrease in strength (299 and 257 N-mm, respectively) as compared
with controls (364 N-mm). Radiographs revealed significantly more
advanced healing in control animals (Goldberg score of 2.1) compared
with the fractures in the rats treated with levofloxacin and trovafloxacin
(Goldberg score of 1.5 in both groups). Fracture calluses in the
animals treated with levofloxacin and trovafloxacin showed a lower
histologic grade (5.3 and 3.5, respectively) as compared with
control animals (7.5) representing a less mature callus with the
presence of more cartilage and less woven bone. These
data suggest that experimental fractures systemically exposed
to levofloxacin or trovafloxacin have diminished healing during
the early stages of fracture repair. The administration of quinolones
during early fracture repair may compromise fracture healing in
humans.
Ref: Levofloxacin and trovafloxacin inhibition
of experimental fracture-healing; by AC Perry et al. Clin Orthop
Relat Res. 2003 Sep;(414):95-100.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12966282
Significant safety issues that have arisen with fluoroquinolones
include phototoxicity, cardiotoxicity, tendinitis, CNS effects
and drug interactions. Ciprofloxacin is well tolerated; the incidence
of adverse events is low and serious adverse events are rare.
Levofloxacin has a reduced CNS adverse event rate compared with
ofloxacin. Sparfloxacin has significant phototoxicity and potential
cardiac toxicity. Grepafloxacin has significantly increased adverse
event rates, particularly gastrointestinal intolerance. Taste
perversion and nausea are common. Trovafloxacin
has an increased potential for CNS adverse reactions, notably
dizziness. Post-marketing surveillance data indicate the possibility
of serious hepatic reactions and pancreatitis. Interactions
between fluoroquinolones and drugs metabolised by the hepatic
cytochrome P450 system affect the clearance of theophylline and
caffeine. Quinolone absorption is significantly reduced by co-administration
of antacids. Hospitalised patients are likely to be receiving
multiple-drug therapy, but drug interactions are avoidable. The
interactions of specific fluoroquinolones should be checked prior
to prescription.
Ref: Safety of the new fluoroquinolones
compared with ciprofloxacin. By P Ball. J Chemother. 2000 Jan;12
Suppl 1:8-11.
|