|
Return to Metaflumizone
Adverse Effects
ACTIVITY:
Insecticide (unclassified)
CAS Name:
2-[2-(4-cyanophenyl)-1-[3-(trifluoromethyl)phenyl]ethylidine]-N-[4-
(trifluoromethoxy)phenyl]hydrazinecarboxamide
US EPA
tolerances: mixture
comprising E- and Z-Isomer:
4-(2E)-2-([4-(trifluoromethoxy)
anilino]carbonylhydrazono)-2-[3-(trifluoromethyl) phenyl]ethylbenzonitrile
and 4-(2Z)-2-([4-(trifluoromethoxy)anilino]carbonyl hydrazono)-2-[3-(trifluoromethyl)
phenyl]ethylbenzonitrile
IUPAC Name:
(EZ)-2'-[2-(4-cyanophenyl)-1-(a,a,a-trifluoro-m-tolyl)ethylidene]-4-(trifluoromethoxy)carbanilohydrazide
EPA
lists the chemical name for
BAS 320 I as:
4-{(2E)-2-({[4-(trifluoromethoxy)anilino]carbonyl{time}
hydrazono)-2-[3-(trifluoromethyl)phenyl]ethyl{time}
benzonitrile - CAS
No. 139968-49-3
and
4-{(2Z)-2-({[4-(trifluoromethoxy)anilino]carbonyl{time}
hydrazono)-2-
[3-(trifluoromethyl) phenyl]ethyl{time} benzonitrile -
CAS
No. 139968-49-3
Structure
for CAS Name:
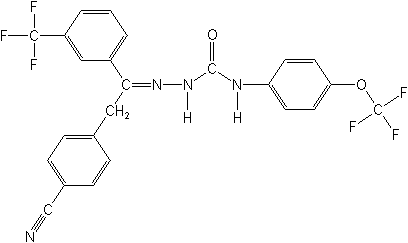
| Adverse
Effects:
Ataxia
Blood
Body Weight Decrease
Bone
Clastogenic
Liver
Reproductive |
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Pending |
| Other
Information |
| Molecular
Formula: |
C24H16F6N4O2 |
| Entry
Year: |
October
2004 |
| Other
Name(s): |
BAS
320 I |
| Manufacturer: |
BASF |
| Of
special interest: |
| 2006. Summary
of toxicology data. California EPA. |
| The
public first heard of this pesticide in October 2004 when it
was announced at the website of the Compendium
of Pesticide Common Names. Soon after,
on Oct. 27, BASF petitioned US EPA for pesticide tolerances
on food. |
| US
Federal Register |
| Date
Published |
Docket
Identification Number |
Details |
| May 2, 2007 |
EPA-HQ-OPP-2007-0305 |
Receipt
of Application for Emergency Exemption, Solicitation of Public
Comment.
EPA has received a specific exemption request from the Georgia
Department of Agriculture to use the pesticide metaflumizone
to treat up to 31,000 acres of Brassica
leafy vegetables to control the diamondback moth. The
Applicant proposes the use of a new chemical which has not been
registered by EPA. Therefore, EPA is soliciting public comment,
on or before June 1, 2007, before making the decision whether
or not to grant the exemption. As part of this request, the
Applicant asserts that the available alternative controls are
no longer providing adequate control, and states that resistance
to some of them may be developing. The Applicant claims that
another control chemical is needed to use in rotation with registered
materials, to maintain season long control of the diamondback
moth in these crops, and that without adequate control, significant
economic losses will be suffered. The
Applicant proposes to make no more than 4 applications of metaflumizone,
at a rate of 0.25 lb. active ingredient per acre (no more than
1.0 lbs. a.i. total), on up to 31,000 acres of Brassica leafy
vegetables (including but not limited to cabbage, collard greens,
mustard greens, kale) and turnip greens, in Georgia, for use
year round, resulting in use of up to a total of 31,000 lbs.
a.i. total. |
| Jan
23, 2006 |
EPA-HQ-OPP-2005-0302 |
BASF.
Notice
of Filing of a Pesticide Petition for the Establishment of
Regulations for the Residues of the Insecticide Metaflumizone
in or on Food and Feed Commodities.
New Tolerance. PP 5F6944. BASF
Corporation, P.O. Box 13528, Research Triangle Park, NC 27709,
proposes to establish tolerances for residues of the insecticide
(mixture
comprising (E-
and Z-Isomer) 4-(2E)-2-([4-(trifluoromethoxy)
anilino]carbonylhydrazono)-2-[3-(trifluoromethyl) phenyl]ethylbenzonitrile
and 4-(2Z)-2-([4-(trifluoromethoxy)anilino]carbonyl
hydrazono)-2-[3-(trifluoromethyl) phenyl]ethylbenzonitrile)
in or on
| Commodity |
PPM |
| cotton,
seed |
0.05 |
| cotton,
gin trash |
35.0 |
| cattle
and poultry, meat |
0.05
|
| cattle
and poultry, fat |
0.5
|
| kidney
and liver, meat byproducts |
0.05 |
| milk |
0.05
|
| eggs |
0.1 |
BASF
Analytical Method No. 531/0 was developed to determine residues
of metaflumizone (E- and Z-Isomer) and its metabolites (M320I04
and M320I23), the residues of concern in plants and in crop
matrices. In this method, residues of metaflumizone are extracted
from plant matrices with methanol/water (70:30; v/v) and then
partitioned into dichloromethane. For oily matrices, the residues
are extracted with a mixture of isohexane/acetonitrile (1:1;
v/v). For animal matrices, a method was developed to determine
the residues of metaflumizone (E-and Z-Isomer), the residues
of concern. For clean-up, a liquid/liquid partition against
dichloromethane is used. The final determination of metaflumizone
is performed by using HPLC-MS-MS.
Pursuant
to 40 CFR 180.7(f), a summary of the petition included in
this notice, prepared by the petitioner along with a description
of the analytical method available for the detection and measurement
of the pesticide chemical residues is available on EPA's Electronic
Docket at http://www.regulations.gov/.
To locate this information on the home page of EPA's Electronic
Docket, select ``Quick Search'' and type the OPP docket ID
number EPA-HQ-OPP-2005-0302 for the pesticide petition (as
specified in Unit I.B.1.) in the search field. Once the search
has located the docket, clicking on the ``Docket ID'' will
bring up a list of all documents in the docket for the pesticide
including the petition summary. |
| Oct
27, 2004 |
OPP-2004-0273 |
BASF.
BAS 320 I: Pesticide
tolerance petition 4F6839;
in or on the raw agricultural commodity
| Commodity |
Parts
Per Million |
tuberous
and corm vegetables
(crop subgroup 1-C)
This
group includes:
arracacha •
arrowroot •
artichoke, chinese •
artichoke, jerusalem •
canna, edible •
cassava
•
chayote root •
chufa •
dasheen •
ginger •
leren •
potato •
potato culls •
potato granules flakes •
potato peel, wet •
potato processed potato waste
•
potato, specialty •
sweet potato •
tanier •
turmeric •
yam bean •
yam, true
|
0.05 |
| leafy
vegetables
(crop group 4)
This
group includes:
amaranth, leafy •
arugula •
cardoon •
celery •
celery, chinese •
celtuce •
chervil •
chervil, fresh leaves •
chrysanthemum, edible leaved •
chrysanthemum, garland •
corn salad •
cress, garden •
cress, upland •
dandelion, leaves •
dock •
endive •
fennel, florence •
fennel, florence, fresh leaves and stalk •
kale, sea •
lettuce, head •
lettuce, leaf •
orach •
orach, leaves •
parsley •
parsley, leaves •
purslane, garden •
purslane, winter •
radicchio •
rhubarb •
spinach •
spinach, chinese •
spinach, new zealand •
spinach, vine •
swiss chard •
tampala •
vegetable, leafy •
vegetable, leafy, except brassica, group
|
35 |
head
and stem brassica
(crop subgroup 5-A)
This
group includes:
broccoli •
broccoli, cavalo •
broccoli, chinese •
brussels sprout •
cabbage •
cabbage, chinese mustard •
cabbage, chinese napa •
cauliflower •
cavalo broccolo •
kohlrabi
|
5 |
leafy
brassica greens
(crop subgroup 5-B)
This
group includes:
broccoli raab •
cabbage, chinese bok choy •
collards •
kale •
mizuna •
mustard greens •
mustard spinach •
rape
greens
|
25 |
fruiting
vegetables
(crop group 8)
This
group includes:
chili, postharvest •
eggplant •
groundcherry •
pepino •
pepper •
pepper, bell •
pepper, nonbell •
pepper, nonbell, sweet
•
tomatillo •
tomato •
tomato, concentrated
products •
tomato, dried pomace
•
tomato, paste •
tomato, puree •
tomato, wet pomace •
vegetable, fruiting •
vegetable, fruiting,
group
|
1.0
|
•
Plant metabolism. In three
plant metabolism studies (cabbage, tomato and cotton), the major
component of the residue was BAS 320 I (E-
and Z-isomers). The major degradate
was the ketone, M320I04
and an oxidized and cyclized metabolite,
M320I23, was present
in lesser amounts. These four compounds
were defined as the residues of concern
•
Genotoxicty
... there was a positive result for a statistically
increased number of structurally aberrant metaphases in the
chromosomes, which indicates clastogenic potential under in
vitro conditions, this result was only
observed without metabolic activation cytogenicity study with
V79 cells. ... it
has also been recognized by EPA that more weight should be placed
on in vivo systems than in vitro systems as expressed in the
Agency's weight of evidence for genotoxic evaluation of a chemical
included in the ``Guidelines for Mutagenicity Risk Assessment''
(Federal Register, September 24, 1986, Vol. 51: 34006-34012)
... based on the weight of the
evidence presented above, BAS 320 I does not pose a genotoxic
concern....
•
Reproductive and developmental
toxicity. ...
a 2-generation reproduction toxicity study in Wistar
rats by oral gavage administration. Originally, the highest
dose tested (HDT) by oral gavage was 75 mg/kg b.w./day, which
induced both excessive maternal toxicity
(very high incidences of poor general health in females during
premating, gestation, and lactation; and statistically decreased
food consumption, body weights, and body weight gain) as well
as excessive developmental toxicity (statistically
impaired pup body weights and body weight gain), which
altogether resulted in high pup mortality. Consequently,
a meaningful assessment of the potential reproductive toxicity
of the test compound at this excessively toxic dose level was
not possible. Thereafter, for the next
two successive parental generations of rats, which were originally
derived from the parents treated at 75 mg/kg b.w./day, the HDT
was 50 mg/kg b.w./day. Subsequently,
the no observable adverse effect level (NOAEL) for parental
toxicity was 20 mg/kg b.w./day, based on the following effects
for females at 50 mg/kg b.w./day (HDT for two consecutive generations)
increased incidences of poor general health in females during
premating, gestation, and lactation; 3 of 25 dams with complete
litter losses; and statistically significantly
reduced body weights during premating, gestation, and lactation.
The
NOAEL for offspring/pup toxicity was 20
mg/kg b.w./day, based on a slight increased incidence
of pup mortality at 50 mg/kg b.w./day. Whereas the NOAEL
for fertility in this study was 50 mg/kg b.w./day (HDT
for two generations), the NOAEL for reproductive
performance was considered to be 20 mg/kg b.w./day, based
on 3 of 25 dams with complete litter losses, of which 2 of these
3 dams had indications of poor nursing for their first generation
of pups.
•
In
a developmental (teratology) toxicity study in the Wistar rat,
the results indicated that the NOAEL for
maternal toxicity was 40 mg/kg b.w./day,
based on statistically decreased food consumption and body weight
gains at 120 mg/kg b.w./day (HDT). The NOAEL for fetal (prenatal)
/developmental toxicity was 120 mg/kg b.w./day (HDT).
•
In a developmental (teratology)
toxicity study in the Himalayan rabbit, the results indicated
that the NOAEL for maternal toxicity was
100 mg/kg b.w./day, based on several clinical symptoms of toxicity
(including ataxia and poor general state)
occurring in 4 of 25 does at 300 mg/kg b.w./day, for
which 2 of these 4 does had abortions prior to being sacrificed
early, with a third doe at 300 mg/kg b.w./day being sacrificed
moribund. Similarly, the NOAEL
for fetal (prenatal)/developmental toxicity was 100 mg/kg b.w./day,
based on slightly decreased mean fetal body weights as well
as an increased rate for a certain
skeletal variation, namely incomplete ossification of sternabrae.
•
Chronic
toxicity.
In the Sprague-Dawley rat, treatment by oral gavage with BAS
320 I for a 2-year chronic duration
resulted in dose-related increased incidences of hepatocellular
centrilobular hypertrophy in the livers of males and females
at 60 mg/kg b.w./day and at 300/200 mg/kg b.w./day and hepatocellular
basophilic alteration in males at 60 and 300 mg/kg b.w./day.
(Note:
Beginning the first day of Week 3, the dose level of the high-dose
females was lowered from 300 to 200 mg/kg
b.w./day, due to an adverse effect of
-71% decreased body weight gain as compared
to controls.)
•
In
the beagle dog,
treatment via gelatin capsules with BAS 320 I for a 12-month
chronic duration resulted in reduced body
weight gain and/or decreased food consumption in several dogs
at 30 mg/kg b.w./day and slightly decreased mean MCHC at 30
mg/kg b.w./day ... For BAS 320 I, the lowest NOAEL for chronic
toxic effects is 12 mg/kg b.w./day from the 12-month dog study.
•
Subchronic
toxicity study with Z-Isomer. In
the Sprague-Dawley rat, treatment
by oral gavage with the Z-isomer of
BAS 320 I for a
subchronic (90-day) duration resulted
in impaired body weight gain only in females at the mid-dose
(300 mg/kg b.w./day) and the high-dose (1,000 mg/kg b.w./day),
as compared to controls. Several microscopic changes were observed
in female animals at these two dose levels, but all morphologic
changes were regarded to be indirect effects of the impaired
body weight gain.
•
Secondary residues from meat, milk, and eggs were not included
in this assessment since the proposed crops are only considered
for human consumption with the exception of processed potato
commodities being potentially utilized in animal feed.
Animal
feeding studies were not required on potatoes based on results
of residues of BAS 320 I and its metabolites (M320I04 and M320I23)
in unwashed potatoes.
Following an application rate 18 times the proposed seasonal
rate, residues in potatoes were at or below the limit of quantitation
(LOQ) and thus the proposed tolerance level was set at the LOQ
and no feeding studies were needed. |
|

