|
Return to Adverse
Effects
ACTIVITY:
Herbicide, Plant growth regulator (anilide)
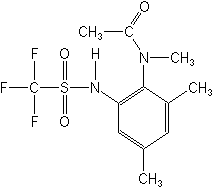
CAS Name:
N-[2,4-dimethyl-5-[[(trifluoromethyl)sulfonyl]amino]phenyl]acetamide
Structure:
| Adverse
Effects:
Body Weight
Decrease
Developmental
Eye
Kidney
Liver
Sciatic nerve
Spleen
Environmental:
• Moderately persistent and mobile in terrestrial environments.
• Above the USEPA's
level of concern for
direct acute (listed and nonlisted) and chronic toxic exposure
to mammals, birds and acute (listed and nonlisted) exposure
to terrestrial and semi aquatic plants.
|
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
Yes |
| US
EPA PC Code: |
114001
(Old US EPA PC Code: 387100) |
| California
Chemical Code |
5082
|
US
EPA Permit Date
and Registrant: |
1978,
3M |
| US
Tolerances: |
Revoked |
Registered
use in
(includes only a limited list of countries)
|
South
Africa, UK, US |
| Other
Information |
| Molecular
Formula: |
C11H13
F3 N2O3S |
| Inventing
Company: |
3M |
| Manufacturers: |
generic |
| Other Names: |
Trade
Name(s):
METHAFLUORIDAMID
MBR 12325
EMBARK PLANT GROWTH REGULATOR
VEL 3973
VISTAR HERBICIDE
S 15017
S 15733
Synonym(s):
ACETAMIDE, N-(2,4-DIMETHYL-5-
(((TRIFLUOROMETHYL)SULFONYL)AMIMO)PHENYL)-
ACETANILIDE, 2',4'-DIMETHYL-5-
((TRIFLUOROMETHYL)SULFONAMIDO)-
TRIFLUOROMETHANESULFONAMIDO)
ACET-2',4'-XYLIDIDE
ACETAMIDO-2,4-DIMETHYLTRIFLUOROMETHANESULFONANILIDE
Scientific Name(s):
DIMETHYL-5-(((TRIFLUOROMETHYL)SULFONYL)AMINO)
PHENYL)ACETAMIDE
Ref:
USEPA/OPP
Chemical Database
|
| Of
special interest: |
| PAN
Data |
2005
- Schedule for Reregistration & Tolerance Reassessment (RED)
for "Mefluidide" is expected
to be December 2007. Contact at EPA: Mark Howard (703) 308-8172;
howard.markt@epa.gov. According to EPA:
Through the pesticide reregistration and tolerance reassessment
programs, EPA is assessing risks and making risk management
decisions for older pesticides. These decisions are summarized
in documents known as REDs, IREDs, and TREDs. By making decisions
according to the schedule below, EPA will meet its statutory
deadlines for completing reregistration and tolerance reassessment.
Some of the decision dates presented in the schedule may change
due to the dynamic nature of the review process. Any pesticide
decisions that are not completed during the current fiscal year
will be rescheduled for the following year. EPA is committed
to meeting its reregistration and tolerance reassessment deadlines.
http://www.epa.gov/pesticides/reregistration/decision_schedule.htm
|
| 1975
- 1993.
US EPA Index of Cleared
Science Reviews |
| October
2001 -
Glossary of Pesticide Chemicals
- A listing of pesticides subject to analysis
of residues in foods and feeds by the US Food and Drug Administration.
|
See
also
Mefluidide, potassiium
salt
Mefluidide,
diethanolamine salt |
| US
Federal Register |
| Date
Published |
Docket
Identification Number |
Details |
|
June 20, 2007
|
EPA-HQ-OPP-2007-0431 |
Mefluidide
Risk Assessments; Notice of Availability. A risk assessment
is being conducted for mefluidide, mefluidide diethanolamine
salt, and mefluidide potassium salt to support the mefluidide
RED. For the purposes of this assessment, all of the three
active ingredients are collectively referred to as mefluidide.
This notice announces the availability of EPA's risk assessments,
and related documents for the pesticide mefluidide, and opens
a public comment period on these documents. The public is
encouraged to suggest risk management ideas or proposals to
address the risks identified. EPA is developing a Reregistration
Eligibility Decision (RED) for mefluidide through a modified,
4-Phase public participation process that the Agency uses
to involve the public in developing pesticide reregistration
decisions. This is Phase 3 of the process.
| Documents in the Federal Register
Docket |
Reader's Guide to Mefluidide
Docket # EPA-HQ-OPP-2007-0431 (June 20, 2007)
EPA-HQ-OPP-2007-0431-0002 |
Mefluidide Use Closure
Memo
EPA-HQ-OPP-2007-0431-0003
• The risk assessments for mefluidide
will be based on the use sites and usage date in BEAD’s
LUIS report, documents presented by the registrants,
and the product labels.
• The two registrants for mefluidide, PBI/Gordon
(technical registrant) and The
Scotts Company (end-use registrant) are supporting
all of the uses for reregistration on their respective
labels.
• The uses that will be included in the reregistration
assessment are; agricultural/farm structures/buildings
and equipment, agricultural/nonagricultural uncultivated
areas/soils, airports/landing fields, commercial industrial
lawns, commercial institutional/industrial premises/equipment
(indoor/outdoor), golf course turf, hospitals/medical
institutions premises (human veterinary), household
domestic dwellings outdoor premises, industrial areas
(outdoor), nonagricultural outdoor buildings/structures,
nonagricultural rights-of-way/fencerows/hedgerows, ornamental
and or shade trees, ornamental ground cover, ornamental
herbaceous plants, ornamental lawns and turf, ornamental
nonflowering plants, ornamental woody shrubs and vines,
paths/patios, paved area (private roads/sidewalks),
recreational areas, and residential lawns. |
Table A3. Non-Food/Non-Feed
Use Patterns Summary for Mefluidide (Case 2370)
EPA-HQ-OPP-2007-0431-0004 |
TABLE A3. NON-FOOD/NON-FEED
USE PATTERNS SUMMARY FOR Mefluidide, diethanolamine
salt (CASE 2370)
EPA-HQ-OPP-2007-0431-0005
|
TABLE A3. NON-FOOD/NON-FEED
USE PATTERNS SUMMARY FOR Potassium mefluidide (CASE
2370)
EPA-HQ-OPP-2007-0431-0006
|
TABLE A3. NON-FOOD/NON-FEED
USE PATTERNS SUMMARY FOR Mefluidide, diethanolamine
salt (CASE 2370)
EPA-HQ-OPP-2007-0431-0007
|
Mefluidide; Diethanolamine
Mefluidide, and Potassium Mefluidide- Phase 2, (30-Day
Error only Correction), HED Chapter of the Re-registration
Eligibility Decision Document (RED).
EPA-HQ-OPP-2007-0431-0008
• There are no agricultural or
any food related pesticide uses of mefluidide. Therefore,
no dietary exposure from food is expected. However,
there is potential for drinking water exposure due to
the outdoor uses of mefluidide.
• Based on the structural similarities of mefluidide
and its diethanolamine (DEA) and potassium salts, where
they all share the same anion- anilide, and the physical
and chemical properties of the DEA and potassium salts,
where they dissociate 100% back to free mefluidide in
aqueous environments, the risk assessment team concluded
that mefluidide DEA and potassium salts are biologically
equivalent to mefluidide and thus they share the same
toxicity as the free mefluidide. Therefore, it is reasonable
to bridge mefluidide toxicity data to mefluidide salts
and vice versa.
• Subchronic and chronic toxicity
of mefluidide is manifested by decreased body weight
and body weight gain in several species tested (rats,
rabbits and dogs). Dogs appeared to be most sensitive
species with the critical toxicological effects of cortical
nephrosis and body weight loss. In rats and rabbits,
critical effects observed were tremors, hunched posture,
salivation, reduced body weight and body weight gain.
• Mefluidide and its diethanolamine
salt subchronic and chronic toxicity are manifested
by decreased body weight and body weight gain in several
species tested (rats, rabbits and dogs). Dogs are most
sensitive to these effects, which occur at doses as
low as 15 mg/kg/day in diets fed for one year. In addition,
dogs fed with mefluidide for one year exhibited chronic
cortical nephrosis at doses of 150 mg/kg/day. Increased
incidence of liver hyperplastic nodules in both sexes
was observed in mice fed with mefluidide at doses of
270 mg/kg/day and higher.
• Mefluidide
or its DEA salt has not been tested for subacute or
subchronic inhalation toxicity. However, both
of them are in category IV for acute inhalation toxicity.
• This risk
assessment relies in part on data from studies in which
adult human subjects were intentionally exposed
to a pesticide or other chemical. These studies (listed
in Appendix B) have been determined to require a review
of their ethical conduct, and have received that review.
• Developmental effects of Mefluidide
in rats included increased number of early resorptions
and mean postimplantation loss. These effects were observed
at the same dose that caused maternal toxicity indicating
there was no increased susceptibility to fetuses. The
maternal toxicity included tremors, decreased body weight,
weight gain and mortality. In rabbit, the LOAEL/NOAEL
for developmental toxicity were above the highest dose
tested (60 mg/kg/day). In the 3-generation reproduction
toxicity study in rats, the offspring toxicity was characterized
by decreased body weights in both sexes and both litters
in all generations.
• After adjusting to the pure
active ingredient, the maternal NOAEL is 58 mg/kg/day
and the LOAEL is 115 mg/kg/day based on clinical signs
(tremors, dark material around the nose, urine stain
and reddish vaginal discharge), decreased body weight
gain, decreased food consumption and mortality (2/25
females). The developmental toxicity NOAEL is also 58
mg/kg/day, the LOAEL is 115 mg/kg/day based on increase
in the number of early resorptions and increase in mean
postimplantation loss.
• . Evidence
of Neurotoxicity. Acute and subchronic neurotoxicity
studies were not performed. Clinical signs of neurotoxicity
(such as tremors, ataxia, atonia, decreased limb tone,
salivation) were seen in several studies (14-day oral
in rabbit at or above 200 mg/kg/day, demyelination study
in chickens at 1000 mg/kg/day and two developmental
toxicity studies in rats at 115 mg/kg/day. Edema and
swelling with myelin loss in sciatic nerve was observed
in a dermal toxicity study in rabbits at doses of 720
mg/kg and above. However, these effects were not seen
in an additional dermal test of similar duration using
a 58.2% mefluidide formulation or diethanolamine salt
of mefluidide 28.8%.
• Environmental
Degradation. The only identified degradation
product was 5-amino-2, 4-dimethyltrifluoromethanesulfonilide.
It was found at a maximum daily concentration of 2.8%
of applied dose (MRID 43162201, aerobic soil). The risk
assessment team concluded that this degradate is not
of concern based on its structure (structurally similar
to the parent, there fore it is not likely to be significantly
more toxic than the parent), and the fact that it is
a minor degradate (<10% of the applied dose). The
residue of concern for drinking water assessment is
parent only.
• . The following acceptable studies
are available:
- Developmental toxicity studies in rats
- Developmental toxicity studies in rabbits
- Two-generation reproduction study in rats |
Mefluidide - Toxicology
section for the Reregistration Eligibility Decision
Document (RED) (January 31, 2007)
EPA-HQ-OPP-2007-0431-0009
|
Mefluidide –
Occupational and Residential Exposure and Risk Assessment
for the Reregistration Eligibility Decision (RED)
EPA-HQ-OPP-2007-0431-0010 |
Appendix A 1-3 Standard
Formulas Used for Calculating Occupational and Residential
Exposures to Mefluidide; Ocupational Handler Exposure
Data and;Risk Calculations for Mefluidide; Residential
Handler
EPA-HQ-OPP-2007-0431-0011
|
Appendix B –
Mefluidide Occupational Handler Risks
EPA-HQ-OPP-2007-0431-0012 |
Appendix C –
Residential Handler Risks
EPA-HQ-OPP-2007-0431-0013 |
Appendix D –
Residential Turf Post Application Risk Assessment for
Melfuidide
EPA-HQ-OPP-2007-0431-0014 |
Mefluidide Incident Report
EPA-HQ-OPP-2007-0431-0015
|
Error Corrections First
Phase for Reregistration of Mefluidide acid, Mefluidide-DEA
and Mefluidide-K (June 9, 2007)
EPA-HQ-OPP-2007-0431-0016 |
Re-registration Eligibility
Document Environmental Fate and Effects Science Chapter
(June 9, 2007) (162
pages)
EPA-HQ-OPP-2007-0431-0017 |
Drinking Water
Assessment for N-[2,4-dimethyl-5-[[(trifluoromethyl)sulfonyl]amino]phenyl]acetamide
(Mefluidide), N-{2,4-dimentyl-5-]]trifluoromethyl)sulfonyl]amino]phenyl’acetamide
monopotassium salt (Potassium Mefluidide), and N-[2,4-dimethyl-5[[(trifluoromethyl)salfonyl]amino]phenyl]actamide
Compound with 2,2’-iminobsi[ethanol] (1:1) (May
9, 2007)
EPA-HQ-OPP-2007-0431-0018 |
|
| Aug
4, 1997 |
OPP-300523 |
Pesticides
Subject to Tolerance Reassessment. |
|
May 15, 1996 |
OPP-34093 |
Mefluidide
and Salts. US EPA's Reregistration Eligibility Decision (RED)
Development Schedule. |
| June
7, 1995 |
na |
Mefluidide,
Potassium Salt ( PBI/Gordon). Request to Delete Use of "Embark
1-L"for utility rights-of-way and roads. |
|