|
Return to Index
Page
ACTIVITY: Herbicide
(Triazolone)
Scientific
Name: 4,5-
dihydro-3-methoxy-4-methyl-5-oxo-N-[[2- (trifluoromethoxy)phenyl]sulfonyl]-1H-1,2,4-triazole-1-carboxamide,
sodium salt
Flucarbazone-sodium
breakdown product: 2-(Trifluoromethoxy)benzenesulfonamide
(metabolite of MKH 6562) (CAS No. 37526-59-3)
N-desmethyl
degradate:
4,5-dihydro-3-methoxy-5- oxo-N-[[2-( trifluoromethoxy)phenyl]sulfonyl]-1H-1,2,4-triazole-1-
carboxamide
Structure
for Flucarbazone:
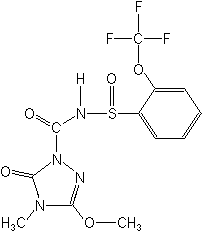
| |
| Date
Pubished |
Docket
Identification Number |
Details |
| December 22, 2006 |
EPA-HQ-OPP-2006-0935 |
Arysta LifeScience.
Pesticide
tolerance. FINAL
RULE. This regulation establishes
a tolerance for combined residues
of flucarbazone-sodium, 4,5-dihydro-3-methoxy-4-methyl-5-oxo-N-[[2(trifluoromethoxy)phenyl]
sulfonyl-1H-1,2,4-triazole 1-carboxamide, sodium salt and
its N-desmethyl metabolite in or on
-- wheat, forage at 0.30 ppm
-- wheat, grain at 0.01 ppm
-- wheat, hay at 0.10 ppm
-- wheat, straw at 0.05 ppm;
and combined residues of flucarbazone-sodium and its metabolites
converted to 2-(trifluoromethoxy) benzene sulfonamide and
calculated as flucarbazone-sodium in or on
-- milk at 0.005 ppm
-- meat and meat byproducts (excluding liver) of cattle, goats,
hogs, horses, and sheep at 0.01 ppm
-- liver of cattle, goats, hogs, horses, and sheep at 1.5
ppm. |
| October 20, 2006 |
EPA-HQ-OPP-2006-0789 |
Arysta
LifeScience.
Pesticide
petition: 6F7112.
New Tolerance proposes to establish a tolerance for residues
of flucarbazone sodium: in or on the raw agricultural commodities
(RACs):
-- Wheat, forage at 0.30 ppm
-- Wheat, grain at 0.01 ppm
-- Wheat, hay at 0.10 ppm
-- Wheat, straw at 0.05 ppm
and combined residues of flucarbazone-sodium and its metabolites
converted to 2-(trifluoromethoxy)benzene sulfonamide and calculated
as flucarbazone-sodium in or on the RACs:
-- Milk at 0.005 ppm
-- Meat and meat byproducts except liver (cattle, goats, sheep,
horses, hogs) at 0.01 ppm
-- Liver (cattle, goats, sheep, horses, hogs) at 1.50 ppm |
| Nov 9,
2005 |
OPP-2005-0254 |
Arysta
LifeScience.
Time-Limited Pesticide
Tolerance. FINAL RULE. The
time limited-tolerance previously issued September 29, 2000,
will be extended for 13 months and will expire on November
30, 2006.
A time-limited tolerance will be issued
due to outstanding studies (independent laboratory validations
of: Analytical Method for the Determination O-Desmethyl
MKH 6562 (Metabolite of MKH 6562 in Soil by High Performance
Liquid Chromatography Tandem Mass Spectrometry), Analytical
Method for the Determination of MKH 6562 and Metabolites NODT
(N,O-dimethyltriazolinone), Sulfonic Acid and Sulfonamide
in Soil by High Performance Liquid Chromatography Tandem Mass
Spectrometry, and Analytical Method for the Determination
of MKH 6562 and Three Metabolites in Groundwater by High Performance
Liquid Chromatography Tandem Mass Spectrometry) will
be submitted to the Agency by the registrant in January 2006.
•
A
summary of the toxicological endpoints for flucarbazone-sodium
used for human risk assessment is discussed in final rule
published in the Federal
Register of September 29, 2000 -
see below.
•
the estimated environmental concentrations (EECs) of flucarbazone-sodium
for acute exposures are estimated to
be 1.42 parts per billion (ppb) or surface water and 0.2
ppb for ground water. The EECs for chronic exposures are estimated
to be 1.25 ppb for surface water and 0.2 ppb for ground water.
•
A default Maximum Residue Limit (MRL)
of 0.01 ppm has been established in Canada for residues of
flucarbazone-sodium and its N-desmethyl metabolite on wheat
grain. This value is consistent with the
tolerance being established in the United States on wheat
grain.
Sec.
180.562 Flucarbazone-sodium; tolerances for residues.
(a) General. (1) Time-limited tolerances are established
for combined residues of the herbicide flucarbazone-sodium
and its N-desmethyl metabolite in or on the following
food commodities: |
| Commodity
|
Parts
per million |
Expiration/Revocation
Date |
| Wheat,
forage |
0.30 |
11/30/06 |
| Wheat,
grain |
0.01 |
11/30/06 |
| Wheat,
hay |
0.10 |
11/30/06 |
| Wheat,
straw |
0.05 |
11/30/06 |
| (2)
Time-limited tolerances are established for combined residues
of the herbicide flucarbazone-sodium and
its metabolites converted to 2-(trifluoromethoxy)benzene
sulfonamide and calculated as flucarbazone-sodium
in or on the following food commodities: |
| Commodity
|
Parts
per million |
Expiration/Revocation
Date |
| Cattle,
liver |
1.50 |
11/30/06 |
| Cattle,
meat |
0.01 |
11/30/06 |
| Cattle,
meat byproducts except liver. |
0.01 |
11/30/06 |
| Goat,
liver |
1.50 |
11/30/06 |
| Goat,
meat |
0.01 |
11/30/06 |
| Goat,
meat byproducts except liver. |
0.01 |
11/30/06 |
| Hog,
liver |
1.50 |
11/30/06 |
| Hog,
meat |
0.01 |
11/30/06 |
| Hog,
meat byproducts except liver. |
0.01 |
11/30/06 |
| Horse,
liver |
1.50 |
11/30/06 |
Horse,
meat |
0.01 |
11/30/06 |
| Horse,
meat byproducts except liver. |
0.01 |
11/30/06 |
| Milk |
0.005 |
11/30/06 |
| Sheep,
liver |
1.50 |
11/30/06 |
| Sheep,
meat |
0.01 |
11/30/06 |
| Sheep,
meat byproducts except liver. |
0.01 |
11/30/06 |
|
| August
3, 2005 |
OPP-2005-0201 |
Cancellation
of Pesticides for Non-payment of Year 2005 Registration Maintenance
Fees.
| Section
24(c) Registrations canceled for non-payment of the
2005
maintenance fee are shown in the following Table 1:
Table
1.--Section 24(c) Registrations Canceled for Non-Payment
of Maintenance Fee |
|
SLN no. |
Product
Name |
| 066330
ID-04-0005 |
Everest
70% Water Dispersible Granular Herbicide |
| 066330
MN-04-0004 |
Everest
70% Water Dispersible Granular Herbicide |
| 066330
ND-04-0002 |
Everest
70% Water Dispersible Granular Herbicide |
| 066330
WA-04-0025 |
Everest
70% Water Dispersible Granular Herbicide |
|
| July
27, 2005 |
OPP-2005-0139 |
Arvesta
Corporation - Pesticide
Petition: PP 5F6949. Petition to establish pesticide tolerance.
The
proposed tolerance expression is parent flucarbazone-sodium
and N-desmethyl flucarbazone-sodium.
•
An
acute neurotoxicity screening study in rats established an
overall NOAEL for males and females of 500 mg/kg based on
transient neurobehavioral effects.
•
Reproductive
and developmental toxicity. Himalayan
rabbits were administered flucarbazone-sodium at levels of
0, 100, 300, 500, or 1,000 mg/kg/bwt by oral gavage days 6
through 28 post coitum in a test for developmental toxicity.
A maternal NOAEL of 100 mg/kg bwt/day was established based
on clinical findings, body weight loss, decreased feed consumption,
gastrointestinal changes, increased liver weights, and fatty
liver changes at 300 mg/kg bwt/day. The
gestation rate NOAEL of 100 mg/kg bwt/day was based on one
abortion (assessed
as secondary due to maternal toxicity) at 300 mg/kg bwt/day.
The NOAEL for fetal parameters of 300
mg/kg bwt/day was based on decreased fetal weights and delayed
ossification at 500 mg/kg bwt/day.
•
Subchronic toxicity. A 28-day
dermal rabbit study established a systemic NOAEL of >1,000
mg/kg bwt/day (the dermal limit dose) for males and females.
The local dermal effects, skin thickening, seen at 1,000 mg/kg
were regarded as a result of mechanical friction and of no
toxicological relevance.
--
A 90-day rat feeding study defined a NOAEL
at 250
ppm (17.6 mg/ kg bwt/day) for males and 1,000 ppm (101.7
mg/kg bwt/day) for females
based
on a decreased spleen weight in males
at 1,000 ppm and on immunologic changes at 4,000 ppm in
females.
-- A 90-day feeding study with male
and female B6C3F1 mice established
a NOAEL of 7,000 ppm (equivalent to >2,083, and 3,051
mg/kg bwt/day for males and females, respectively). The
dose of 7,000 ppm was the HDT.
-- A 90-day dog
feeding study at levels of 0, 1,000, 5,000, and 50,000 ppm
established a NOAEL of 1,000 ppm (equivalent to 33.8 mg/kg
bwt/day in males and 35.2 mg/kg bwt/day in females) based
on decreased thyroxine levels and
increased thyroxine-binding capacity, macroscopic and microscopic
effects on the gastric mucosa and an eosinophilic hepatocellular
cytoplasm occurring at 5,000 ppm and above. The liver
enzyme induction at 1,000 ppm was assessed as a slight adaptive
response in the detoxification process of flucarbazone-sodium
but not as an adverse effect, due to the absence of clinical
chemical changes that would indicate liver damage and due
to the absence of any histopathologic liver changes at this
dietary level.
-- A
28-day (6 hours/day; 5 days/week) subacute inhalation toxicity
study was conducted with male and female Wistar rats
exposed
to mean actual concentrations of 5.2, 30.0, 180.1 and 513.3
mg/m3 air. A NOAEL of 5.2 mg/m3 air
was established based on histopathological changes observed
at 30 mg/m3 air and above.
| To
establish a tolerance for residues of flucarbazone-sodium:
4,5-dihydro-3-methoxy-4-methyl-5-oxo-N-[[2-(trifluoromethoxy)phenyl]sulfonyl]-1H-1,2,4-triazole
1-carboxamide, sodium salt; and its N-desmethyl metabolite
in or on the raw agricultural commodities (RACs): |
| Commodity
|
Parts
per million |
| Wheat,
forage |
0.30 |
| Wheat,
grain |
0.01 |
| Wheat,
hay |
0.10 |
| Wheat,
straw |
0.05 |
| And
combined
residues of flucarbazone-sodium and its metabolites converted
to 2-(trifluoromethoxy)benzene sulfonamide and
calculated as flucarbazone-sodium in or on the raw agricultural
commodities: |
| Commodity
|
Parts
per million |
| Milk |
0.005 |
Meat
and meat byproducts except liver
(cattle, goats, sheep, horses, hogs) |
0.01 |
| Liver
(cattle, goats, sheep, horses, hogs) |
1.50 |
|
| Feb
21, 2001 |
OPP-30482B |
- BAYER
- Conditional
Approval of Herbicide Products:
- Everest
Technical, for formulation of herbicides for use on spring
and winter wheat (EPA Reg No. 3125-533);
- Everest
70% Water Dispersible Granular In Water Soluble Packets,
for post emergence control of wild oat and green foxtail
in spring and winter wheat (EPA Reg. No. 3125-534);
- Everest
70% Water Dispersible Granular Herbicide, for post emergence
control of wild oat and green foxtail in spring and winter
wheat (EPA Reg. No. 3125-535).
|
| Sept
29, 2000 |
OPP-301052 |
BAYER
- Time-Limited
Pesticide Tolerances. - FINAL RULE. This regulation
establishes time-limited tolerances for combined residues
of and its N-desmethyl metabolite in or on wheat, forage at
0.30 parts ppm; wheat, grain at 0.01 ppm; wheat, hay at 0.10
ppm; and wheat, straw at 0.05 ppm; and combined residues of
flucarbazone-sodium and its metabolites converted to 2- (trifluoromethoxy)benzene
sulfonamide and calculated as flucarbazone- sodium in or on
milk at 0.005 ppm; meat and meat byproducts (excluding liver)
of cattle, goats, hogs, horses and sheep at 0.01 ppm; and
liver of cattle, goats, hogs, horses and sheep at 1.5 ppm.
The tolerances will expire and be revoked on November 1, 2005. |
| Oct
20, 1999 |
OPP-
30482 |
- BAYER
- Registration
applications for THREE pesticide products:
- Flucarbazone-Sodium
Technical Herbicide.
Active ingredient: Flucarbazone-sodium at 95.6%. For use
only in the manufacturing of herbicides.
- Flucarbazone-Sodium
70% Water Dispersible Granular Herbicide.
Active ingredient: Flucarbazone-sodium at 70%. For post
emergence control of wild oat and green foxtail in all types
of spring wheat.
- Flucarbazone-Sodium
70% Water Dispersible Granular In Water Soluble Packets.
Active ingredient: Flucarbazone-sodium at 70%. For post
emergence control of wild oat and green foxtail in all types
of spring wheat.
|
| Oct
8, 1999 |
PF-894 |
BAYER
- Pesticide
Petition to establish tolerances for residues of flucarbazone
sodium and its N-desmethyl degradate in or on the raw agricultural
commodities:
• WHEAT:
forage at 0.30 ppm
hay at 0.10 ppm
straw at 0.05 ppm
grain at 0.01 ppm
• MILK at 0.005 ppm
• MEAT (cattle, goats, sheep, horses, hogs) at 0.01
ppm
• LIVER (cattle, goats, sheep, horses, hogs) at 0.60
ppm |
|

