|
Return to
Index Page
Adverse
Effects
ACTIVITY:
Acaricide, Ovicide (unclassified)
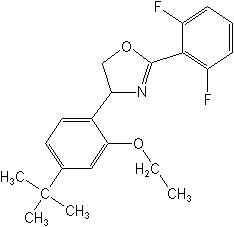
CAS Name: 2-(2,6-difluorophenyl)-4-[4-(1,1-dimethylethyl)-2-ethoxyphenyl]-4,5-dihydrooxazole
Structure:
US
Federal Register |
| Date
Published |
Docket
Identification Number |
Details |
| June 27, 2007 |
EPA-HQ-OPP-2007-0309 |
IR-4.
Pesticide
Petition. PP 6E7150. Proposal to establish a tolerance
for residues of the insecticide etoxazole in or on food commodities:
| hop, dried cones |
7.0 ppm |
melon, subgroup
9A
This subgroup includes
6 commodities.
cantaloupe • citron melon • melon •
melon, citron • muskmelon • watermelon
|
0.15 ppm |
| cherry |
0.70 ppm |
Practical analytical methods for detecting
and measuring levels of etoxazole have been developed and
validated in/on all appropriate agricultural commodities and
respective processing fractions. The LOQ of etoxazole in the
methods is 0.02 ppm which will allow monitoring of food with
residues at the levels proposed for the tolerances. |
| July
20, 2005 |
OPP-2005-0170 |
VALENT.
Pesticide Tolerance.
FINAL RULE.
| Commodity
|
Parts
per million |
| Almond,
hulls |
2.0
ppm |
| Grape |
0.50
ppm |
| Grape,
raisin |
1.5
ppm |
Nut,
tree, group 14
This
group includes 16 commodities.
almond
• almond, hulls • beechnut • butternut
• cashew • chestnut • chinquapin
• filbert • nut, brazil • nut, hickory
• nut, macadamia • nutmeat, processed,
except peanut • nuts • pecan • pistachio
• walnut
|
0.01
ppm |
| Pistachio |
0.01
ppm |
A
summary of the toxicological endpoints for etoxazole used
for human risk assessment is discussed in Unit III.B. of the
final rule published in the Federal
Register of September 26, 2003 (see
below)
•
Prenatal and postnatal sensitivity.
There
is qualitative evidence of increased susceptibility following
exposure to etoxazole in the rat reproduction study.
Therefore, EPA performed a Degree of Concern Analysis to determine
the LOC for the effects observed when considered in the context
of all available toxicity data, and to identify any residual
uncertainties after establishing toxicity endpoints and traditional
UFs to be used in the risk assessment of this chemical. If
residual uncertainties are identified, EPA examines whether
these residual uncertainties can be addressed by a special
FQPA safety factor and, if so, the size of the factor needed.
In performing the Degree of Concern Analysis, EPA noted that
the effects in the pups in the rat reproduction study are
well-characterized with a clear NOAEL. In addition, the pup
effects occur at the same dose as maternal toxicity. Furthermore,
the doses selected for various risk assessment scenarios are
lower than the doses that caused off spring toxicity. There
are no residual uncertainties for prenatal/postnatal toxicity
in this study. Therefore, although there
is evidence of increased qualitative susceptibility in the
rat reproduction study, the concern is low. For the reasons
stated above, EPA has concluded that there is low concern
for prenatal and/or postnatal toxicity resulting from exposure
to etoxazole.
• EPA
determined that the 10X SF (safety factor) to protect infants
and children should be removed.
• Acute exposure... EPA
evaluated the suitability of the developmental
toxicity study in rabbits in which the developmental NOAEL
of 200 milligram/kilogram/day (mg/kg/day) is based upon increased
incidences of 27 presacral vertebrae and 27 presacral vertebrae
with 13th ribs (skeletal variations) in the fetuses at the
LOAEL of 1,000 mg/kg/day (limit dose). Although these
developmental effects may be attributed to a single dose,
EPA concluded that these effects are minor in magnitude and
were observed only at the limit dose (1,000 mg/kg/day).
Therefore,
quantitation of the acute risk was not performed. |
| April
13, 2005 |
OPP-2005-0047 |
VALENT.
Pesticide Petition
to Establish a Tolerance in or on Food:
| Commodity |
PPM |
| nut,
tree (Crop Group 14), including pistachios |
0.01 |
| almond,
hulls |
2.0 |
| grapes |
0.5 |
| raisins |
1.5
|
Nut,
tree Group 14 includes 16 commodities.
almond • almond, hulls • beechnut •
butternut • cashew • chestnut • chinquapin
• filbert • nut, brazil • nut, hickory
• nut, macadamia • nutmeat, processed, except
peanut • nuts • pecan • pistachio •
walnut |
•
The results of a grape processing study indicate that etoxazole
residues concentrate in both grape juice and raisins. The
concentration factor for raisins was determined in this study
to be 3.5X.
The theoretical concentration factor for raisins is, however,
4.7x.
•
Almond, hull is the only commodity
under consideration that is a significant
feed item for beef and dairy cattle.
•
Rabbit
developmental study.
A statistically significant increased incidence of 27 presacral
vertebrae with 13th ribs was observed in fetuses at 1,000
mg/kg/day compared with controls.
This finding was within historical control range for fetal
incidence but above the historical control
range for litter incidence. No dose response was evident
and the variation is considered to be
equivocally treatment related. The NOAEL for developmental
toxicity was 200 mg/kg/day based on statistically
significant increased incidence of 27 presacral vertebrae
with 13th ribs in fetuses at 1,000 mg/kg/day.
•
Subchronic
toxicity. Subchronic
toxicity studies conducted with etoxazole technical in the
rat (oral and dermal), mouse and dog
indicate a low level of toxicity.
Effects observed at high dose levels consisted primarily of
anemia and histological changes in the adrenal gland, liver
and kidneys.
•
Rat
chronic feeding/oncogenicity study.
Sprague Dawley rats for 2-years at dietary concentrations
of 4, 16, and 64 mg/kg/day. A trend toward decreased body
weight gain for males at 64 mg/kg/day in the latter half of
the study was observed. Hemotology and
clinical chemistry changes, increased liver weights and hepatic
enlargement at 16 mg/kg/day or above were observed. Testicular
masses, centrilobular hepatocellular swelling and testicular
interstitial (Leydig) cell tumors occurred at or above 16
mg/kg/day. The interstitial (Leydig) cell tumors were
believed to be incidental. The NOAEL was 4 mg/kg/day for males
and 16 mg/kg/day for females. Because
an MTD level was not achieved in this study, a second study
was conducted in which etoxazole technical was fed
to male and female Sprague Dawley rats for 2-years at dietary
concentrations of 50, 5,000, and 10,000 ppm. In this study,
decreased mortality, body weight and food consumption/ efficiency
(females) at 10,000 ppm was observed.
Hematological, clinical, and histopathological changes of
the incisors, and increased liver weights occurred in both
sexes at 5,000 and 10,000 ppm. Centrilobular hepatocellular
hypertrophy was observed in both sexes at 10,000 ppm. The
interstitial (Leydig) cell tumors observed in the first study,
were not observed in the repeat study. The NOAEL in
the repeat study was 50 ppm (1.8 mg/kg/day).
•
Animal
metabolism... Plasma
levels were significantly lower in females. Concentrations
of radioactivity were significantly higher in the tissues
of male rats compared to females.
... By 168 hours, the concentration in most tissues was below
the concentration in the corresponding plasma, with only
the liver and fat having significant
levels of radioactivity.
|
| Sept
26, 2003 |
OPP-2003-0289 |
VALENT.
Pesticide tolerance.
FINAL RULE.
Commodity
(ppm)
Apple, wet pomace.......................0.50
Cattle, fat ....................................0.02
Cattle, liver ..................................0.01
Cotton, gin byproducts .................1.0
Cotton, undelinted seed ................0.05
Fruit, pome, group 11 [see below]
..0.20
Goat, fat ......................................0.02.
Goat, liver ....................................0.01
Horse, fat ....................................0.02.
Horse, liver ..................................0.01
Milk, fat .......................................0.01
Sheep, fat ....................................0.02
Sheep, liver ..................................0.01
Strawberry ...................................0.50
Tangerine\1\ .................................0.10 |
| \1\There
are no U.S. registrations for use of etoxazole on tangerines
as of September 26, 2003. |
| Fruit,
pome, group 11:
apple
apple, dried pomace
apple, juice
apple, wet pomace
crabapple |
fruit,
pome
loquat
mayhaw
pear
pear, oriental
quince |
-- 90-Day
oral toxicity rodents (rat).
NOAEL = 61.8/69.0 milligrams/kilogram/ day (mg/kg/day) Male/Female
(M/F) LOAEL = 183.7/204.8 mg/kg/day (M/F), based upon increases
in hepatic enzyme levels, increased
liver weights and centrilobular hepatocellular swelling
in both sexes and liver enlargement
in females only
-- 90-Day oral toxicity rodents (mouse).
NOAEL = 213.6/250.5 mg/kg/day (M/F) LOAEL = 878.4/994.5 mg/kg/day
(M/F), based upon periportal hepatocellular
necrosis, increased alkaline phosphatase levels, accompanied
by increased relative liver weight,
liver enlargement, and centrilobular hepatocellular swelling
-- 90-Day oral toxicity nonrodents (dog).
NOAEL = 5.33/5.42 mg/ kg/day (M/F) LOAEL = 53.7/55.9 mg/ kg/day
(M/F), based upon clinical signs (vomiting foamy fluid and
mucous stool), clinical chemistry,
increased liver weights, and centrilobular swelling in the
liver and acinar cell atrophy
in the prostate.
-- Prenatal developmental toxicity in nonrodents (rabbit).
Maternal NOAEL = 200 mg/kg/day LOAEL = 1,000 mg/kg/day based
upon liver enlargement and
decreased body weight gains and
food consumption. Developmental
NOAEL = 200 mg/kg/day LOAEL = 1,000 mg/kg/ day based upon
increased incidences of 27 presacral
vertebrae and 27 presacral vertebrae with 13th ribs in the
fetuses.
-- Reproduction and fertility effects
(rat). Parental/Systemic NOAEL
= 20 mg/kg/ day LOAEL = 100 mg/kg/ day (M/F), based upon increased
liver weights in the P and F1
males and increased adrenal weights in the P females
Offspring/Systemic NOAEL = 20 mg/kg/ day LOAEL = 100 mg/kg/
day (M/F), based upon pup mortality
Reproductive NOAEL = 100 mg/kg/day LOAEL = not determined.
-- Combined chronic toxicity/ carcinogenicity rodents (rat).
NOAEL = 64 mg/kg/day (M/F). LOAEL = not determined Equivocal
evidence of carcinogenicity.
-- 2-Year feed/ carcinogenic (rat)
. NOAEL = 1.83/2.07 mg/kg/day (M/F) LOAEL = 187/216 (M/ F),
based upon effects on the incisors including abnormal
amelogenesis. No evidence of carcinogenicity
-- Chronic toxicity nonrodents (dog).
NOAEL = 4.62/4.79 mg/kg/day (M/F). LOAEL = 23.5/23.8 mg/ kg/day
(M/F), based upon increased alkaline phosphatase activity,
increased liver weights, liver enlargement
(females), and incidences of centrilobular hepatocellular
swelling in the liver.
-- 78-Week carcinogenic mouse. NOAEL = 242/243 (M/ F). LOAEL
= 484/482 (M/ F), based on a slight increase in the incidence
of a fatty change in the centrilobular
hepatocytes in males.
-- Gene mutation -
in vitro forward gene mutation assay in mouse lymphoma cells.
When tested up to cytotoxic levels, mutagenic in the
presence of S9 activation and equivocal
for mutagenicity in the absence of S9 activation.
-- Cancer. EPA has determined that etoxazole
is not likely to be a human carcinogen and EPA therefore,
does not expect it to pose a cancer risk. As a result,
a quantitative cancer dietary exposure analysis was not performed. |
| Aug
3, 2003 |
OPP-2003-0271 |
VALENT.
Pesticide tolerance petition.
| Commodity |
Requested
Residue Tolerances |
| pome
fruit (Crop Group 11)
apple
apple, dried pomace
apple, juice
apple, wet pomace crabapple
fruit, pome
loquat
mayhaw
pear
pear, oriental
quince |
0.2
ppm |
| apple
wet pomace |
1.0
ppm |
| strawberry
|
0.5
ppm |
| cottonseed
|
0.05
ppm |
| cotton,
gin byproducts (gin trash) |
1.0
ppm |
| oranges |
0.10
ppm
(to
support the importation of mandarin oranges into
the U.S.) |
| As
residues in processed commodities fed to animals may be
transferred to milk and edible tissue of ruminants, tolerances
are also proposed for |
| animal
fat |
0.03
ppm |
| milk
fat |
0.04 ppm |
--
Maximum Residue Limits for etoxazole have been established to
allow the following uses of etoxazole in the following countries:
Turkey, Israel, South Africa, Japan, France, Taiwan, and Korea.
The use pattern and MRL's are similar to those proposed for
the U.S.
-- The toxicology reports for etoxazole have not yet been reviewed
by EPA and thus, the Agency has not yet established toxic endpoints
of concern, specifically chronic and acute oral toxicity endpoints
for the compound.
-- Genotoxicty... Etoxazole produced a positive result in the
mouse lymphoma gene mutation assay but only in the presence
of metabolic activation.
-- Rabbit developmental study. Etoxazole technical was administered
by oral gavage to pregnant rabbits at dosage levels of 40, 200,
and 1,000 mg/kg/day on days 6 through 18 of gestation.
Decreased body weight, body weight gain, food consumption and
enlarged liver were noted at 1,000 mg/kg/ day...
A
statistically significant increased incidence of 27 presacral
vertebrae with 13th ribs was observed in fetuses at 1,000 mg/kg/day
compared with controls.
This finding was within historical control range for fetal incidence
but above the historical control range
for litter incidence...
-- Rat reproduction study... The parental NOAEL was 400 ppm
(17.0 mg/ kg/day) based on the effects on relative
liver weight in males at 2,000 ppm. The pup NOAEL was
400 ppm (37.9 mg/kg/day) based on decreased
viability on lactation Day 4 and decreased body weight at 2,000
ppm in the F1 pups.
-- Subchronic toxicity. Effects observed at high dose levels
consisted primarily of anemia and histological
changes in the adrenal gland, liver and kidneys.
-- Rat feeding study. The NOAEL was 100 ppm for males and 300
ppm for females based on increased incidence
of hepatocellular swelling at 1,000 ppm and 3,000 ppm.
-- Mouse feeding study. The NOAEL was 400
ppm for males and 1,600 ppm for females based on increased
alkaline phosphatase activity, increased liver weights, and
increased incidence of hepatocellular swelling at 6,400
ppm (both sexes) and at 1,600 ppm in males
and enlarged livers in females at 6,400 ppm.
-- Dog feeding study. he NOAEL was 200 ppm (5.3 mg/kg/day) based
on clinical signs, clinical pathology
changes, liver weight effects and histopathological changes
at 2,000 and 10,000 ppm.
-- Chronic toxicity. Valent proposes a chronic oral endpoint
of 4 mg/kg bwt/day, based on the NOAEL for male rats in a two-
year chronic toxicity oncogenicity feeding study.
-- Dog chronic feeding study. The NOAEL was 200 ppm (4.6 mg/kg/day
for males and 4.79 mg/kg/day for females) based on increased
absolute and relative liver weights with corresponding histopathological
changes in the liver at 1,000 and 5,000 ppm.
-- Rat chronic feeding/oncogenicity study. In the first study,
etoxazole technical was fed to male and female Sprague Dawley
rats for two years at dietary concentrations of 4, 16, and 64
mg/kg/day. A trend toward decreased body
weight gain for males at 64 mg/kg/day in the latter half of
the study was observed. Hemotology and clinical chemistry changes,
increased liver weights and hepatic enlargement at 16 mg/kg/day
or above were observed. Testicular masses, centrilobular hepatocellular
swelling and testicular interstitial (Leydig) cell tumors occurred
at or above 16 mg/kg/day. The interstitial (Leydig) cell tumors
were believed to be incidental. The NOAEL was 4 mg/kg/day for
males and 16 mg/kg/day for females.
-- Endocrine disruption. No special studies to investigate the
potential for estrogenic or other endocrine effects of etoxazole
have been performed. |
|