|
Return to
Index Page
Adverse
Effects
ACTIVITY: Herbicide
(2,6-Dinitroaniline)
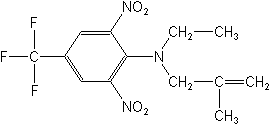
CAS NAME:
N-ethyl-N-(2-methyl-2-propenyl)-2,6-dinitro-4-(trifluoromethyl)benzenamine
Structure:
| |
| Date
Published |
Docket
Identification Number |
Details |
| August
31, 2005 |
OPP-2005-0195 |
Dow
AgroSciences & IR-4.
Pesticide Tolerance
Petition. Petitions: PP
1E6326, PP 2E6360 and PP 2E6466 for
the raw agricultural commodities:
| Commodity |
Part
Per Million |
| canola |
0.05
|
| crambe |
0.05
|
| mustard
seed |
0.05
|
| potato |
0.05 |
| rapeseed |
0.05
|
| dill |
0.05 |
•
The summary of the petition was prepared by Interregional
Research Project Number 4 (IR-4). IR-4 submitted the
petitions on behalf of the registrant, Dow
AgroSciences LLC, who prepared this notice of filing.
•
Genotoxicty.
Ethalfluralin was weakly mutagenic in activated strains TA1535
and TA100 of salmonella typhimurium, but not in strains TA1537,
TA1538, and TA98 in an Ames assay... In Chinese hamster ovary
cells, ethalfluralin was negative without S9 activation, but
it was clastogenic with activation.
• Reproductive and developmental
toxicity. The developmental NOAEL in rats was 1,000
mg/kg/day, the highest dose. In rabbits
the NOAELs for maternal and developmental toxicity were 75
mg/kg/day. The maternal LOAEL at 150 mg/kg/day was based on
abortions and decreased food consumption.
These effects as well as decreased
weight gain, enlarged liver, and orange urine were found at
300 mg/kg/day. In this study developmental toxicity was observed.
The developmental LOAEL in rabbits was
150 mg/kg/day, based on slightly increased resorptions,
abnormal cranial development, and increased sternal variants.
In a three-generation rat reproduction study, the parental
NOAEL was 12.5 mg/kg/day. The parental LOAEL was 37.5 mg/kg/day,
based on depressed mean body weight
gains in males in all generations. No treatment-related
effects were noted on reproductive parameters and the NOAEL
was 37.5 mg/kg/day or greater. A 7-month multigeneration bridging
study was conducted with doses equivalent to 0, 8, 20, or
61 mg/kg/day in the
diet of Fischer 344 rats. The parental NOAEL was 20 mg/kg/day.
The parental LOAEL was 61 mg/kg/day based on
increased liver weights.
•
Chronic toxicity.
Ethalfluralin was administered to Fisher
344 rats in the diet for 2 years in combined chronic toxicity
and carcinogenicity replicate studies.
The doses were equivalent to 0, 4.2, 10.7, or 32.3
mg/kg/day. The NOAEL for systemic effects was 32.3 mg/kg/day.
Mammary gland fibroadenomas were found in dosed female rats
at statistically significant incidences in the mid and high
doses. EPA's Office of Pesticide Program's
Carcinogenicity Peer Review Committee concluded that, ethalfluralin
should be classified as Group C, a possible human carcinogen,
based on increased mammary gland fibroadenomas and adenomas/fibroadenomas
combined in female rats. The tumor incidences were statistically
significant at both the mid and high dose, and exceeded the
upper range of historical controls.
Based on a low dose extrapolation, the Q1* of 8.9 x 10-2 (mg/kg/day)-1
has been calculated. Based on both registered and proposed
product uses, exposure to ethalfluralin from food is estimated
to not exceed a lifetime cancer risk of 8.47 x 10-7. Cancer
risks of less than 1 x 10-6 are generally considered to be
negligible.
• Beagle dogs were given 0, 4,
20, or 80 mg/kg/day orally, by capsule, for 1 year. The NOAEL
was 4 mg/kg/day. The LOAEL was 20 mg/kg/day, based on increased
urinary bilirubin, variations in erythrocyte morphology, increased
thrombocyte count, and increased erythroid series of the bone
marrow. Elevated alkaline phosphatase levels were found at
the two higher doses and siderosis of the liver at the high
dose. |
| July
31, 2002 |
OPP-2002-0155
|
Tolerance Revocations.
FINAL RULE. When EPA establishes tolerances for residues
in or on raw agricultural commodities, consideration must be
given to the possible residues of those pesticides in meat,
milk, poultry, and/or eggs produced by animals that are fed
agricultural products (for example, grain or hay) containing
pesticide residues (40 CFR 180.6). When considering this possibility,
the EPA can conclude that (1) finite residues
will exist in meat, milk, poultry, and/or eggs; (2) there
is a reasonable expectation that finite residues will exist;
or (3) there is a reasonable expectation that finite residues
will not exist. In 1994, the ethalfluralin
RED recommended revocation for egg, milk, fat, meat, and meat
byproduct tolerances based on animal metabolism data
(submitted since the time that the tolerances were originally
established) from which EPA concluded that there is no reasonable
expectation of finite residues for meat, fat, and meat byproduct
commodities and the associated tolerances are not required according
to 40 CFR 180.6(a)(3). Those feeding studies used exaggerated
amounts of the pesticide and did not show measurable residues
in animal tissues. Therefore, the Agency
is revoking the tolerances in 40 CFR 180.416 for residues of
ethalfluralin in or on goats, fat; goats, meat; and goats, mbyp.
|
| April
15, 2002 |
OPP-2002-0019 |
Proposed
Revocation of Tolerances. "When
EPA establishes tolerances for residues in or on raw agricultural
commodities, consideration must be given to the possible residues
of those pesticides in meat, milk, poultry, and/or eggs produced
by animals that are fed agricultural products (for example,
grain or hay) containing pesticide residues (40 CFR 180.6).
When considering this possibility, EPA can conclude that:
(1) Finite residues will exist in meat, milk, poultry, and/or
eggs; (2) there is a reasonable expectation that finite residues
will exist; or (3) there is a reasonable expectation that
finite residues will not exist. In 1994, the ethalfluralin
RED recommended revocation for egg, milk, fat, meat, and meat
byproduct tolerances based on animal metabolism data (submitted
since the time that the tolerances were originally established)
from which EPA concluded that there is no reasonable expectation
of finite residues for meat, fat, and meat byproduct commodities
and the associated tolerances are not required according to
40 CFR 180.6(a)(3). Those feeding studies used exaggerated
amounts of the pesticide and did not show measurable residues
in animal tissues. Since the ethalfluralin RED, completed
prior to the implementation of the FQPA, the Agency has reviewed
the regulatory conclusions in the RED and determined in a
memorandum January 3, 2002, that for egg, milk, fat, meat,
and meat byproduct commodities there is no reasonable expectation
of finite residues and the egg, milk, fat, meat, and meat
byproduct tolerances for ethalfluralin are no longer needed
and are not required according to 40 CFR 180.6(a)(3). Therefore,
the Agency is proposing to revoke the tolerances in 40 CFR
180.416 for residues of ethalfluralin in or on goats, fat;
goats, meat; and goats, mbyp. A copy of the Agency's January
3, 2002 memorandum will be placed in the docket." |
| Jan
17, 2002 |
OPP-301208
|
IR-4
* - Pesticide
Tolerance of 0.05 ppm in or on Canola and Safflower seed.-
FINAL RULE.
Prenatal / developmental non-rodent studies: Abnormal cranial
development, increase in sternal variants.
Chronic Toxicity in Dogs: increased urinary bilirubin, variations
in erythrocyte morphology, increased thrombocyte count, and
increased erythroid series of the bone marrow.
Combined Chronic/Carcinogenicity rat studies: Mammary gland
fibroadenomas were found in dosed female rats at statistically
significant incidences in mid and high doses.
Combined Chronic / Carcinogenicity mice studies: focal hepatocellular
hyperplasia in both sexes and increased liver, kidney, and
heart weights in females.
Bacterial reverse mutation test: weakly mutagenic.
In vitro mammalian chromosome aberration test: clastogenic
with S9 activation.
2-year chronic carcinogenicity rat study: increased incidence
of mammary gland fibroadenomas and combined adenomas/ fibroadenomas
in female rats. |
| Nov
14, 2001 |
PF-1052 |
IR-4
* - Three
pesticide petitions to establish tolerances for residues in
or on Canola, Safflower and Dill at 0.05 ppm. |
| Nov
14, 2001 |
OPP-181082 |
3
Pesticide Emergency Exemptions. EPA authorized the use in:
Minnesota: on canola to control kochia; April 13, 2001 to December
31, 2001. Montana & North Dakota: on canola to control kochia;
April 13, 2001 to December 31, 2001. |
| Aug
8, 2001 |
OPP-301155 |
Emergency
Exemptions for tolerances in or on safflower seed at 0.05 ppm.
- FINAL RULE. Comments on this proposal must be received
by EPA on or before October 9, 2001. |
| July
19, 2001 |
OPP-301146 |
Extension
of Tolerances for Emergency Exemptions. EPA has authorized
the use of ethalfluralin on canola for control of kochia in
Montana, Minnesota, and North Dakota. This regulation extends
a time-limited tolerance for residues of the herbicide ethalfluralin
in or on canola at 0.05 ppm for an additional 2-year period.
This tolerance will expire and is revoked on Decmber 31, 2003.
|
| Dec
20, 2000 |
OPP-181078 |
Pesticide
Emergency Exemptions. EPA authorized the use of ethalfluralin
on Canola from 4/7/00 to 4/7/01 in Minnesota, Montana, and North
Dakota. |
| Oct
8, 1999 |
OPP-300925 |
Reestablishment
of Tolerance for Emergency Exemptions. - FINAL RULE.
This regulation reestablishes a time-limited tolerance for residues
of the herbicide ethalfluralin and its metabolites in or on
canola at 0.05 ppm for an additional 2-year period. This tolerance
will expire and is revoked on December 31, 2001. |
| July
26, 1999 |
na |
Completion
of Comment Period for Reregistration Eligibility Decision (RED)
Document. NTIS Publication No. 738-R-95-001; EPA Case No.
2260; RED Date, March 1995. |
| Feb
11, 1998 |
na |
PLATTE
CHEMICAL - Request
to Voluntarily Cancel 2 Pesticide Registrations for Clean
Crop Curbit EC Herbicide. EPA Reg. Nos. 034704 NH-94-0001
and 034704 OH-91-0004. |
| Dec
17, 1997 |
OPP-300585 |
Pesticide
Tolerances for Canola Seed. Emergency Exemption. - FINAL
RULE. This regulation establishes a time-limited tolerance
for residues of ethalfluralin in or on safflower seed. The tolerance
will expire and is revoked on October 31, 1998. |
| Aug
4, 1997 |
OPP-300523 |
Pesticides
Subject to Tolerance Reassessment. |
| May
15, 1996 |
OPP-34093 |
EPA's
Reregistration Eligibility Decision (RED) Development Schedule. |
| March
20, 1996 |
OPP-34091 |
Availability
of Reregistration Eligibility Decision (RED) for comment. |
| *
Interregional Research Project Number 4 (IR-4) 681
U.S. Highway # 1, South, North Brunswick, NJ 08902- 3390 |
|