|
Return
to Thiazopyr Index Page
Activity:
Herbicide
(Pyridine
carboxylic acid)
Structure:
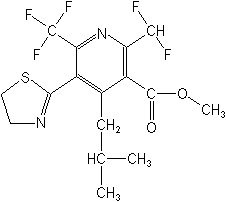
Adverse Effects:
Amyloidosis
Blood
Body Weight Decrease
Bone
Cancer: Likely to be a Human Carcinogen -
THYROID,
KIDNEY
Cholesterol
Endocrine: Pituitary (disruption
of the thyroid-pituitary hormonal feedback mechanism)
Endocrine: Suspected Disruptor
Endocrine: Thyroid
Eye
Kidney
Liver
Environmental
•
US: As of February 17, 2005, this herbicide (and its metabolites)
are permitted
in or on 2
food commodities - Grapefruit and Orange -
see list at bottom of page
•
European Commission: Not allowed to be used after July 25,
2003
|
Amyloidosis
(click
on for all fluorinated pesticides)
-- A mouse carcinogenicity
study at doses of 0, 0.17, 1.6, 16.9, 66.3 or 128.4 mg/kg/day
(males) and 0,0.24, 2.6, 26.8, 108.1 or 215.9 mg/kg/day (female)
with a systemic NOEL of 0.1 mg/kg/day. The effects were hepatocellular
hypertropy and amyloid deposition.
At 66.3 mg/kg/day the same lesions
plus increased liver weights, random
and periportal hepatocellular vacuolation
were observed. At 128.4 mg/kg/day the
same lesions plus distended abdonen, slight increase
in ALP, SGOT and SGPT, abnormal coloration
and enlargement of liver, decrease
in absolute and relative spleen weights,
increase in absolute and relative kidney
weights, increase in eosinophilia
in hepatocytes, kidney nephropathy and lymphocytic hyperplasia
of the nesenteric lymph nodes were observed. There was no evidence
of oncoenicity at any dose level.
Ref: US EPA. Pesticide Fact Sheet. Thiazopyr
Reason for Issuance: Registration of a New Chemical Date Issued:
February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Blood
(click
on for all fluorinated pesticides)
-- Thiazopyr technical
produced organ toxicity following multiple exposures at high doses.
The primary target organs for thiazopyr toxicity in the rat, mouse
and dog were the liver, thyroid, kidney
and blood, with the
liver being the most sensitive indicator of toxicity. In
chronic dietary feeding studies, the dog was the most sensitive
species. An RfD for thiazopyr of 0.008 mg/kg/day was established
by the RfD Committee of the USEPA Health Effects Division, based
on the NOEL of 0.8 mg a.i./kg/day (20 ppm) from the chronic dog
study and a 100-fold safety factor to account for intraspecies
extrapolation and intraspecies variability.
-- 90-day Oral (Rat): NOEL (systemic) =100 ppm (6.60 mg /kg/day
and 7.99 mg/kg/day for males and females, respectively). The LOEL
was 1000 ppm (68 - 79 mg/kg/day in males and females, respectively)
based on increased liver, thyroid and kidney
weights, changes in clinical chemistry and hematological
parameters and on gross and microscopic
changes observed in the liver and thyroid at does levels of 68
mg/kg/day and higher. At the 201 mg/kg/day dose diffused thyroid
follicular cell hypertrophy/ hyperplasia was observed.
-- 90-day Oral (Dog): NOEL (systemic) =10 ppm. (0.2 mg/kg/day(m);
0.3 mg/kg/day(f)), based on decreased body
weight gain and increased SGPT [serum
glutamic-oxaloacetic transaminase] levels at 3 and 6 m/kg/day
for males and females, respectively and above; decreased total
protein and albumin concentration and albumin/globulin ratio,
increased AP, hepatocytic hypertrophy, oval cell proliferation
and increased hepatocytic fatty content at 35 mg/kg/day and above;
and decreased calcium concentration which is thought to be related
to hypoalbuminemia, decreased cholesterol
and triglyceride concentrations, slightly increased GGT and SGPT,
follicular hyperplasia of thyroid, increased colloid content in
follicles and increased relative thyroid weight at 175 mg/kg/day.
-- A 1 year feeding study in dogs at 0, 0.8, 7.8, 86.0 with males,
and 0.8, 8.8, and 78.0 with females with a NOEL of 0.8 mg/kg/day.
The Loel was based on hepatocellular hypertrophy and hyperplasia.
A 10% increase in prothrombin time and several and several
changes in blood chemistry: increased
SGOT, SGPT, GGT and ALK levels and
decreased cholesterol, albumin and total protein and calcium were
observed in high- dose dogs. There were increases in absolute
weights, liver and body weight and liver to brain weight,
heptotoxicity characterized by enlargement and/or discoloration
in some high dose animals and by hepatocellular hypertrophy/hyperplasia
in the 0.8 and 7.8 mg/kg/day dogs. The NOEL was based on hepatocellular
hypertrophy and hyperplasia.
-- A two year rat carcinogenicity study at doses of 0, 0.04, 4.4,
44.2 or 136.4 mg/kg/day (Males) 0, 0.06, 0.6, 5.6, 56.3 or 177.1
mg/kg/day (female) with a NOEL of 4.4 mg/kg/day. The effects were
protruding eyes, evidence of mild anemia, increased
GGT and cholesterol,
increased absolute and relative liver, kidney
and thyroid weights and significant increase in microscopic lesions
in the liver (hypertrophy and vacuolar changes), kidney (nephropathy)
and thyroid (hypertrophy and hyperplasia); decreased mean body
weight and body weight gain and food consumption. A statistically
significant increase in thyroid follicular cell adenomas/cystadenomas
were observed in males at 44.2 and 136.4 mg/kg/day. A nonsignificant
increase in renal tubular adenomas in high-dose females
was considered to be equivocal.
Ref: US EPA. Pesticide Fact Sheet. Thiazopyr
Reason for Issuance: Registration of a New Chemical Date Issued:
February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Body
Weight Decrease
(click
on for all fluorinated pesticides)
-- 90-day Oral (Dog):
NOEL (systemic) =10 ppm. (0.2 mg/kg/day(m); 0.3 mg/kg/day(f)),
based on decreased body weight gain
and increased SGPT
[serum glutamic-oxaloacetic transaminase] levels at 3 and
6 m/kg/day for males and females, respectively and above; decreased
total protein and albumin concentration and albumin/globulin ratio,
increased AP, hepatocytic hypertrophy, oval cell proliferation
and increased hepatocytic fatty content at 35 mg/kg/day and above;
and decreased calcium concentration which is thought to be related
to hypoalbuminemia, decreased cholesterol and triglyceride concentrations,
slightly increased GGT and SGPT, follicular hyperplasia of thyroid,
increased colloid content in follicles and increased relative
thyroid weight at 175 mg/kg/day.
-- A developmental toxicity study in rats at 0, 10, 100 and 250
mg/kg/day with a maternal toxicity NOEL of 100 mg/kg/day. The
effect were increased liver
weight, increased salivation, significantly
decreased body weight gain and decreased food consumption.
The developmental NOEL was also 100 mg/kg/day. The effects at
the high dose were increased incidence of
unossified sternebrae and 7th cervical rib variation. No development
effects were observed below the maternally toxic doses.
-- A two year rat carcinogenicity study at doses of 0, 0.04, 4.4,
44.2 or 136.4 mg/kg/day (Males) 0, 0.06, 0.6, 5.6, 56.3 or 177.1
mg/kg/day (female) with a NOEL of 4.4 mg/kg/day. The effects were
protruding eyes, evidence of mild anemia,
increased GGT and cholesterol, increased absolute and relative
liver, kidney and thyroid weights and significant increase in
microscopic lesions in the liver (hypertrophy and vacuolar changes),
kidney (nephropathy) and thyroid (hypertrophy and hyperplasia);
decreased mean body weight and body weight
gain and food consumption.
A statistically significant increase in
thyroid follicular cell adenomas/cystadenomas were observed in
males at 44.2 and 136.4 mg/kg/day. A nonsignificant increase
in renal tubular adenomas in high-dose females was considered
to be equivocal.
-- a. Thiazopyr was administered through the diet at 0 and 150
mg/kg/day rats to determine the subchronic effect on hormone level
and other biochemical endpoints. Animals were assayed at 7, 14,
28, 56 or 90 days. Significant decreases
in body weight gain were observed at 90 days. Early
in the study the treated rats showed increases in TSH (ranging
from 133 to 200% of controls) and decreases in T4 (ranging from
43% to 76% of controls). In addition there were increases in liver
and thyroid weights and increases in thyroid follicular cell hypertrophy/hyperplasia.
Reverse T3 was increased at 28 days, and T3 was either not affected
or increased. There were indications of increases in hepatic UDPGT
activity and significant increases in T4 UDPGT activity. Hepatic
5'-monodeiodinase activity was either not affected or decreased.
The effects observed in this study were supportive of the theory
that thiazopyr may induce thyroid tumors through a disruption
in the thyroid-pituitary hormonal feedback mechanisms.
Ref: US EPA. Pesticide Fact Sheet. Thiazopyr
Reason for Issuance: Registration of a New Chemical Date Issued:
February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Bone
(click
on for all fluorinated pesticides)
-- A developmental
toxicity study in rats at 0, 10, 100 and 250 mg/kg/day with a
maternal toxicity NOEL of 100 mg/kg/day. The effect were increased
liver weight, increased salivation, significantly
decreased body weight gain and decreased food consumption. The
developmental NOEL was also 100 mg/kg/day. The effects at the
high dose were increased incidence of unossified
sternebrae and 7th cervical rib variation. No development
effects were observed below the maternally toxic doses.
Ref: US EPA. Pesticide Fact Sheet. Thiazopyr
Reason for Issuance: Registration of a New Chemical Date Issued:
February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Cancer:
Likely to be a Human Carcinogen - THYROID, KIDNEY
(click
on for all fluorinated pesticides)
Group
C -- Possible Human Carcinogen. Statistically
significant increase in thyroid follicular
cell tumors (M). Increases in renal
tubular adenomas (M & F); however statistically significant
positive trend in F only; Sprague-Dawley rats.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Group C--Possible Human Carcinogen.
Reviewed 5/ 25/ 94.
Ref: List of Chemicals Evaluated
for Carcinogenic Potential. Science Information Management Branch,
Health Effects Division, Office of Pesticide Programs, U. S. Environmental
Protection Agency. March 15, 2002.
http://www.biomuncie.org/chemicals_evaluated_for_carcinog.htm
--
The thyroid tumors were determined
in three special thyroid function studies to be secondary to a
disturbance of thyroid/pituitary
homeostasis and were attributed to a hormonally-mediated
mechanism for thyroid tumor induction.
The effects were dose-responsive and with the exception of thyroid
weight, all effects were completely reversible when thiazopyr
was removed from the diet. Based on limited evidence for carcinogenicity,
thiazopyr is classified as Category
C, possible human carcinogen, by the USEPA Health Effects Division
Carcinogenicity Peer Review Committee. A NOEL of 4.4 mg/kg/day
and a Margin of Exposure approach were selected for use in carcinogenicity
risk assessment...
-- A two year rat carcinogenicity study at doses of 0, 0.04, 4.4,
44.2 or 136.4 mg/kg/day (Males) 0, 0.06, 0.6, 5.6, 56.3 or 177.1
mg/kg/day (female) with a NOEL of 4.4 mg/kg/day. The effects were
protruding eyes, evidence of
mild anemia, increased GGT and cholesterol, increased absolute
and relative liver, kidney and thyroid weights and significant
increase in microscopic lesions in the liver (hypertrophy and
vacuolar changes), kidney (nephropathy) and thyroid (hypertrophy
and hyperplasia); decreased mean body weight and body weight gain
and food consumption. A statistically
significant increase in thyroid follicular cell adenomas/cystadenomas
were observed in males at 44.2 and 136.4 mg/kg/day. A nonsignificant
increase in renal tubular adenomas in high-dose females was considered
to be equivocal.
-- The EPA Health Effects Division Carcinogenicity
Peer Review Committee classified thiazopyr as a Group C, possible
human carcinogen and recommended that for the purpose of risk
characterization a Margin of Exposure (M.O.E.) approach should
be used in evaluation of the consequences of human exposure.
-- Special mechanistic studies for mode of toxic action on thyroid
function. The results of three studies on the effects of thiazopyr
on thyroid function and mechanisms involved in the disposition
of T4 in rats were reviewed. These studies are described below:
---- a. Thiazopyr was administered through the diet at 0 and 150
mg/kg/day rats to determine the subchronic effect on hormone level
and other biochemical endpoints. Animals were assayed at 7, 14,
28, 56 or 90 days. Significant decreases in body weight gain were
observed at 90 days. Early in the study the treated rats showed
increases in TSH (ranging from 133 to 200% of controls) and decreases
in T4 (ranging from 43% to 76% of controls). In addition there
were increases in liver and thyroid weights and increases in thyroid
follicular cell hypertrophy/hyperplasia. Reverse T3 was increased
at 28 days, and T3 was either not affected or increased. There
were indications of increases in hepatic UDPGT activity and significant
increases in T4 UDPGT activity. Hepatic 5'-monodeiodinase activity
was either not affected or decreased. The effects observed in
this study were supportive of the theory that thiazopyr may induce
thyroid tumors through a disruption in the thyroid-pituitary hormonal
feedback mechanisms.
---- b. A second study on the effects of thiazopyr on the biochemical
mechanisms of thyroid toxicity in rats at doses of 0, 0.5, 1.5,
5, 15, 50 or 150 mg/kg/day was conducted. Dose response effects
on various biochemical parameters were observed. Two groups of
the rats in the study were observed for reversibility of effects
observed up to 56 and 112 days. Doses at
15, 50 and 150 mg/kg/day significantly increased the liver weights.
Thyroid weights were increased at doses of 50 and 150 mg/kg/day.
There were no significant effect on body weight or body weight
gains during the study. The T4 UDPGT levels
were increased by 117 and 376% above controls at the 50 and 150
mg/kg/day dosages. Effects of 150 mg/kg/day were increases
in T3, TSH and rT3 serum concentrations, and increased incidence
of follicular cell hypertrophy/hyperplasia at the 150 mg/kg/day
dose. A NOEL of 1.5 mg/kg/day was determined based on liver weight
increases. Thyroid weight was the only parameter that did not
return to those similar to the controls.
At the 56 and 112 day recovery periods the thyroid weights were
120 and 123% of control values, respectively.
---- c. A third thyroid function study on the biochemical mechanisms
involved with disposition of T4 in rats fed dosages of 0 and 150
mg/kg/day for 56 days was conducted. Rats feed thiazopyr had
increase T4 UDPGT activity and total deiodinase activity in their
livers. There was also a two-fold increase in mixed function oxidase
enzyme activity.
-- Results of the three studies suggest that increased glucuronidation,
deiodination of T4 and T3, and increased rate of clearance of
T4 from the blood and excretion of the hormone and its metabolites
in the bile could significantly reduce the level of circulating
T4 in the male rat. Results of these studies support the hypothesis
tht thiazopyr may induce thyroid tumors through a disruption of
the thyroid-pituitary hormonal feedback mechanism circulating
T4 in the male rat.
Ref: US EPA. Pesticide Fact Sheet.
Thiazopyr Reason for Issuance: Registration of a New Chemical
Date Issued: February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Cholesterol
(click
on for all fluorinated pesticides)
-- A two year rat carcinogenicity study at doses of 0, 0.04,
4.4, 44.2 or 136.4 mg/kg/day (Males) 0, 0.06, 0.6, 5.6, 56.3 or
177.1 mg/kg/day (female) with a NOEL of
4.4 mg/kg/day. The effects were protruding eyes, evidence of mild
anemia, increased GGT and cholesterol,
increased absolute and relative liver, kidney and thyroid weights
and significant increase in microscopic lesions in the liver (hypertrophy
and vacuolar changes), kidney (nephropathy) and thyroid (hypertrophy
and hyperplasia); decreased mean body weight and body weight gain
and food consumption. A statistically significant increase in
thyroid follicular cell adenomas/cystadenomas were observed in
males at 44.2 and 136.4 mg/kg/day. A nonsignificant increase in
renal tubular adenomas in high-dose females was considered to
be equivocal.
Ref: US EPA. Pesticide
Fact Sheet. Thiazopyr Reason for Issuance: Registration of a New
Chemical Date Issued: February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Endocrine:
Pituitary
(click
on for all fluorinated pesticides)
-- There was no evidence
of carcinogenic effects in an 18-month chronic/oncogenicity study
in mice at dose levels up to and including 800 ppm (216 mg/kg/day).
In rats, an increased incidence of thyroid
follicular tumors in males at the two highest doses, 1000 (44.2
mg/kg/day, males) and 3000 ppm, (136.4 mg/kg/day) was observed,
and there was a low incidence of renal tubular adenoma at the
high dose only in females. The thyroid
tumors were determined in three special thyroid function studies
to be secondary to a disturbance of thyroid/pituitary homeostasis
and were attributed to a hormonally-mediated
mechanism for thyroid tumor induction.
The effects were dose-responsive and with the exception of thyroid
weight, all effects were completely reversible when thiazopyr
was removed from the diet. Based on limited evidence for carcinogenicity,
thiazopyr is classified as Category C, possible human carcinogen,
by the USEPA Health Effects Division Carcinogenicity Peer Review
Committee. A NOEL of 4.4 mg/kg/day
and a Margin of Exposure approach were selected for use
in carcinogenicity risk assessment.
-- Special mechanistic studies for mode of toxic action on
thyroid function. The results of three studies on the effects
of thiazopyr on thyroid function and mechanisms involved
in the disposition of T4 in rats were reviewed. These studies
are described below:
---- a. Thiazopyr was administered through
the diet at 0 and 150 mg/kg/day rats to determine the subchronic
effect on hormone level and other biochemical endpoints. Animals
were assayed at 7, 14, 28, 56 or 90 days. Significant decreases
in body weight gain were observed at 90 days. Early in the study
the treated rats showed increases in TSH (ranging from 133 to
200% of controls) and decreases in T4 (ranging from 43% to 76%
of controls). In addition there were increases in liver and thyroid
weights and increases in thyroid follicular cell hypertrophy/hyperplasia.
Reverse T3 was increased at 28 days, and T3 was either not affected
or increased. There were indications of increases in hepatic UDPGT
activity and significant increases in T4 UDPGT activity. Hepatic
5'-monodeiodinase activity was either not affected or decreased.
The effects observed in this study were supportive of the theory
that thiazopyr may induce thyroid
tumors through a disruption in the thyroid-pituitary hormonal
feedback mechanisms.
---- b. A second study on the effects of thiazopyr on the biochemical
mechanisms of thyroid toxicity in rats at doses of 0, 0.5, 1.5,
5, 15, 50 or 150 mg/kg/day was conducted. Dose response effects
on various biochemical parameters were observed. Two groups of
the rats in the study were observed for reversibility of effects
observed up to 56 and 112 days. Doses at 15, 50 and 150 mg/kg/day
significantly increased the liver weights. Thyroid weights were
increased at doses of 50 and 150 mg/kg/day. There were no significant
effect on body weight or body weight gains during the study. The
T4 UDPGT levels were increased by 117 and 376% above controls
at the 50 and 150 mg/kg/day dosages. Effects of 150 mg/kg/day
were increases in T3, TSH and rT3 serum concentrations, and increased
incidence of follicular cell hypertrophy/hyperplasia at the 150
mg/kg/day dose. A NOEL of 1.5 mg/kg/day was determined based on
liver weight increases. Thyroid weight was the only parameter
that did not return to those similar to the controls. At the 56
and 112 day recovery periods the thyroid weights were 120 and
123% of control values, respectively.
---- c. A third thyroid function study on the biochemical mechanisms
involved with disposition of T4 in rats fed dosages of 0 and 150
mg/kg/day for 56 days was conducted. Rats feed thiazopyr had increase
T4 UDPGT activity and total deiodinase activity in their livers.
There was also a two-fold increase in mixed function oxidase
enzyme activity.
-- Results of the three studies suggest
that increased glucuronidation, deiodination of T4 and T3, and
increased rate of clearance of T4 from the blood and excretion
of the hormone and its metabolites in the bile could significantly
reduce the level of circulating T4 in the male rat. Results of
these studies support the hypothesis tht thiazopyr may induce
thyroid tumors through a disruption of the
thyroid-pituitary hormonal feedback mechanism circulating T4 in
the male rat.
Ref: US EPA. Pesticide Fact Sheet. Thiazopyr
Reason for Issuance: Registration of a New Chemical Date Issued:
February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Endocrine:
Suspected Disruptor
(click
on for all fluorinated pesticides)
Suspected
Endocrine Disruptor
Ref: June 14, 2001 - Implementation of the
Community Strategy for Endocrine Disruptors - a range of substances
suspected of interfering with the hormone systems of humans and
wildlife. Communication from the Commission to the Council and
the European Parliament. Commission of the European Communities,
Brussels COM (2001) 262 final.
http://www.fluoridealert.org/pesticides/Endocrine.Disruptors.EC2001.pdf
- More information available at:
http://europa.eu.int/eur-lex/en/com/cnc/2001/com2001_0262en01.pdf
Endocrine:
Thyroid (click
on for all fluorinated pesticides)
-- There was no evidence of carcinogenic effects in an 18-month
chronic/oncogenicity study in mice at dose levels up to and including
800 ppm (216 mg/kg/day). In rats, an increased incidence of thyroid
follicular tumors in males at the two highest doses, 1000
(44.2 mg/kg/day, males) and 3000 ppm, (136.4 mg/kg/day) was observed,
and there was a low incidence of renal tubular
adenoma at the high dose only in females. The
thyroid tumors were determined in three special thyroid function
studies to be secondary to a disturbance of thyroid/pituitary
homeostasis and were attributed to a hormonally-mediated mechanism
for thyroid tumor induction. The effects were dose-responsive
and with the exception of thyroid weight,
all effects were completely reversible when thiazopyr was removed
from the diet. Based on limited evidence for carcinogenicity,
thiazopyr is classified as Category C, possible human carcinogen,
by the USEPA Health Effects Division Carcinogenicity Peer Review
Committee. A NOEL of 4.4 mg/kg/day and a Margin of Exposure
approach were selected for use in carcinogenicity risk assessment.
-- Thiazopyr technical produced organ toxicity following multiple
exposures at high doses. The primary target organs for thiazopyr
toxicity in the rat, mouse and dog were the
liver, thyroid, kidney
and blood, with the liver
being the most sensitive indicator of toxicity. In chronic
dietary feeding studies, the dog was the most sensitive species.
An RfD for thiazopyr of 0.008 mg/kg/day was established by the
RfD Committee of the USEPA Health Effects Division, based on the
NOEL of 0.8 mg a.i./kg/day (20 ppm) from the chronic dog study
and a 100-fold safety factor to account for intraspecies extrapolation
and intraspecies variability.
-- 90-day Oral (Rat): NOEL (systemic) =100 ppm (6.60 mg /kg/day
and 7.99 mg/kg/day for males and females, respectively). The LOEL
was 1000 ppm (68 - 79 mg/kg/day in males and females, respectively)
based on increased liver,
thyroid and kidney weights,
changes in clinical chemistry and hematological parameters and
on gross and microscopic changes observed
in the liver and thyroid at
does levels of 68 mg/kg/day and higher. At the 201 mg/kg/day dose
diffused thyroid follicular cell hypertrophy/
hyperplasia was observed.
-- 90-day Oral (Dog): NOEL (systemic) =10 ppm. (0.2 mg/kg/day(m);
0.3 mg/kg/day(f)), based on decreased body
weight gain and increased SGPT levels at 3 and 6 m/kg/day for
males and females, respectively and above; decreased total protein
and albumin concentration and albumin/globulin ratio, increased
AP, hepatocytic hypertrophy, oval cell proliferation and
increased hepatocytic fatty content at 35 mg/kg/day and above;
and decreased calcium concentration which is thought to be related
to hypoalbuminemia, decreased cholesterol and triglyceride concentrations,
slightly increased GGT and SGPT, follicular
hyperplasia of thyroid, increased colloid content in follicles
and increased relative thyroid weight
at 175 mg/kg/day.
-- A 3 week dermal study in rabbits at 0, 100, 500 and 1000 mg/kg/day
with a NOEL of 100 mg/kg/day. The effects were increased mean
absolute and relative kidney weights and minimal multifocal or
periportal hypatocyte vacuolation.
-- A two year rat carcinogenicity study at doses of 0, 0.04, 4.4,
44.2 or 136.4 mg/kg/day (Males) 0, 0.06, 0.6, 5.6, 56.3 or 177.1
mg/kg/day (female) with a NOEL of 4.4 mg/kg/day. The effects were
protruding eyes, evidence of mild anemia,
increased GGT and cholesterol, increased absolute and relative
liver, kidney and thyroid weights
and significant increase in microscopic
lesions in the liver (hypertrophy and vacuolar changes), kidney
(nephropathy) and thyroid (hypertrophy
and hyperplasia); decreased mean
body weight and body weight gain and food consumption. A
statistically significant increase in thyroid
follicular cell adenomas/cystadenomas were observed in males at
44.2 and 136.4 mg/kg/day. A nonsignificant
increase in renal tubular adenomas in high-dose females was considered
to be equivocal.
-- The EPA Health Effects Division Carcinogenicity Peer Review
Committee classified thiazopyr as a Group
C, possible human carcinogen and recommended that for the
purpose of risk characterization a Margin of Exposure (M.O.E.)
approach should be used in evaluation of the consequences of human
exposure.
-- Special mechanistic studies for mode of toxic action on thyroid
function. The results of three studies on the effects of
thiazopyr on thyroid function and
mechanisms involved in the disposition of T4 in rats were reviewed.
These studies are described below:
---- a. Thiazopyr was administered through the diet at 0 and 150
mg/kg/day rats to determine the subchronic effect on hormone level
and other biochemical endpoints. Animals were assayed at 7, 14,
28, 56 or 90 days. Significant decreases
in body weight gain were observed at 90 days. Early in
the study the treated rats showed increases in TSH
(ranging from 133 to 200% of controls) and decreases
in T4 (ranging from 43% to 76% of controls). In addition
there were increases in liver and thyroid
weights and increases in thyroid
follicular cell hypertrophy/hyperplasia. Reverse T3 was
increased at 28 days, and T3 was either not affected or increased.
There were indications of increases in hepatic
UDPGT activity and significant increases
in T4 UDPGT activity. Hepatic 5'-monodeiodinase activity
was either not affected or decreased. The effects observed in
this study were supportive of the theory that thiazopyr may induce
thyroid tumors through a disruption in the
thyroid-pituitary hormonal feedback mechanisms.
---- b. A second study on the effects of thiazopyr on the biochemical
mechanisms of thyroid toxicity in rats at doses of 0, 0.5, 1.5,
5, 15, 50 or 150 mg/kg/day was conducted. Dose response effects
on various biochemical parameters were observed. Two groups of
the rats in the study were observed for reversibility of effects
observed up to 56 and 112 days. Doses at 15, 50 and 150 mg/kg/day
significantly increased the liver weights.
Thyroid weights were increased at
doses of 50 and 150 mg/kg/day. There were no significant effect
on body weight or body weight gains during the study. The T4 UDPGT
levels were increased by 117 and 376% above controls at the 50
and 150 mg/kg/day dosages. Effects of 150 mg/kg/day were increases
in T3, TSH and rT3 serum concentrations, and increased incidence
of follicular cell hypertrophy/hyperplasia
at the 150 mg/kg/day dose. A NOEL of 1.5 mg/kg/day was determined
based on liver weight increases. Thyroid
weight was the only parameter that did not return to those similar
to the controls. At the 56 and 112 day recovery periods the thyroid
weights were 120 and 123% of control values, respectively.
---- c. A third thyroid function study on the biochemical mechanisms
involved with disposition of T4 in rats fed dosages of 0 and 150
mg/kg/day for 56 days was conducted. Rats feed thiazopyr had increase
T4 UDPGT activity and total deiodinase
activity in their livers. There was also a two-fold increase in
mixed function oxidase enzyme activity.
-- Results of the three studies suggest that increased glucuronidation,
deiodination of T4 and T3, and increased
rate of clearance of T4 from the
blood and excretion of the hormone and its metabolites in the
bile could significantly reduce the level of circulating T4
in the male rat. Results of these studies support the hypothesis
tht thiazopyr may induce thyroid tumors
through a disruption of the thyroid-pituitary hormonal feedback
mechanism circulating T4 in the male rat.
Ref: US EPA. Pesticide Fact Sheet. Thiazopyr
Reason for Issuance: Registration of a New Chemical Date Issued:
February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Eye
(click on for all
fluorinated pesticides)
-- It is considered
to be moderately irritating to the skin and substantially
irritating to the eye.
-- A two year rat carcinogenicity study at doses of 0, 0.04, 4.4,
44.2 or 136.4 mg/kg/day (Males) 0, 0.06, 0.6, 5.6, 56.3 or 177.1
mg/kg/day (female) with a NOEL of 4.4 mg/kg/day.
The effects were protruding eyes,
evidence of mild anemia, increased GGT and cholesterol, increased
absolute and relative liver, kidney and thyroid weights and significant
increase in microscopic lesions in the liver (hypertrophy and
vacuolar changes), kidney (nephropathy) and thyroid (hypertrophy
and hyperplasia); decreased mean body weight and body weight gain
and food consumption. A statistically significant increase in
thyroid follicular cell adenomas/cystadenomas were observed in
males at 44.2 and 136.4 mg/kg/day. A nonsignificant increase in
renal tubular adenomas in high-dose females was considered to
be equivocal.
Ref: US EPA Pesticide Fact Sheet.
February 20, 1997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Kidney
(click
on for all fluorinated pesticides)
Group
C -- Possible Human Carcinogen. Statistically
significant increase in thyroid follicular
cell tumors (M). Increases in renal
tubular adenomas (M & F); however statistically significant
positive trend in F only; Sprague-Dawley rats.
Ref: April
26, 2006 . Chemicals Evaluated for Carcinogenic Potential by the
Office of Pesticide Programs. From: Jess Rowland, Chief Science
Information Management Branch Health Effect Division (7509C) Office
of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
-- A two year rat carcinogenicity
study at doses of 0, 0.04, 4.4, 44.2 or 136.4 mg/kg/day (Males)
0, 0.06, 0.6, 5.6, 56.3 or 177.1 mg/kg/day (female) with a NOEL
of 4.4 mg/kg/day. The effects were protruding
eyes, evidence of mild anemia, increased
GGT and cholesterol, increased absolute and relative liver, kidney
and thyroid weights and significant increase in microscopic lesions
in the liver (hypertrophy and vacuolar changes), kidney
(nephropathy) and thyroid (hypertrophy and hyperplasia);
decreased mean body weight and body weight gain and food consumption.
A statistically significant increase in thyroid follicular cell
adenomas/cystadenomas were observed in males at 44.2 and 136.4
mg/kg/day. A nonsignificant increase
in renal tubular adenomas in high-dose females was considered
to be equivocal.
-- Thiazopyr technical produced organ toxicity following multiple
exposures at high doses. The primary target
organs for thiazopyr toxicity in the rat, mouse and dog
were the liver, thyroid, kidney
and blood,
with the liver being the most sensitive indicator of toxicity.
In chronic dietary feeding studies, the dog was the most
sensitive species. An RfD for thiazopyr of 0.008 mg/kg/day was
established by the RfD Committee of the USEPA Health Effects Division,
based on the NOEL of 0.8 mg a.i./kg/day (20 ppm) from the chronic
dog study and a 100-fold safety factor to account for intraspecies
extrapolation and intraspecies variability.
-- 90-day Oral (Rat): NOEL (systemic) =100 ppm (6.60 mg /kg/day
and 7.99 mg/kg/day for males and females, respectively). The LOEL
was 1000 ppm (68 - 79 mg/kg/day in males and females, respectively)
based on increased liver, thyroid and kidney
weights, changes in clinical chemistry and hematological
parameters and on gross and microscopic changes observed in the
liver and thyroid at does levels of 68 mg/kg/day and higher. At
the 201 mg/kg/day dose diffused thyroid follicular cell hypertrophy/
hyperplasia was observed.
-- A 3 week dermal study in rabbits at 0, 100, 500 and 1000 mg/kg/day
with a NOEL of 100 mg/kg/day. The effects were increased mean
absolute and relative kidney weights
and minimal multifocal or periportal hypatocyte vacuolation.
-- A mouse carcinogenicity study at doses
of 0, 0.17, 1.6, 16.9, 66.3 or 128.4 mg/kg/day (males) and 0,0.24,
2.6, 26.8, 108.1 or 215.9 mg/kg/day (female) with a systemic NOEL
of 0.1 mg/kg/day. The effects were hepatocellular hypertropy and
amyloid deposition. At 66.3
mg/kg/day the same lesions
plus increased liver weights, random and periportal hepatocellular
vacuolation were observed. At 128.4 mg/kg/day the
same lesions plus distended abdonen, slight increase
in ALP, SGOT and SGPT, abnormal coloration and enlargement of
liver, decrease in absolute and relative spleen weights, increase
in absolute and relative kidney
weights, increase in eosinophilia in hepatocytes,
kidney nephropathy and lymphocytic hyperplasia
of the nesenteric lymph nodes
were observed. There was no evidence of oncoenicity at any dose
level.
Ref: US EPA. Pesticide Fact Sheet. Thiazopyr
Reason for Issuance: Registration of a New Chemical Date Issued:
February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
Liver
(click on for all fluorinated
pesticides)
-- Thiazopyr technical
produced organ toxicity following multiple exposures at high doses.
The primary target organs for thiazopyr toxicity in the rat, mouse
and dog were the liver,
thyroid, kidney and blood,
with the
liver being the most sensitive indicator of toxicity. In
chronic dietary feeding studies, the dog was the most sensitive
species. An RfD for thiazopyr of 0.008 mg/kg/day was established
by the RfD Committee of the USEPA Health Effects Division, based
on the NOEL of 0.8 mg a.i./kg/day (20 ppm) from the chronic dog
study and a 100-fold safety factor to account for intraspecies
extrapolation and intraspecies variability.
-- 21-Day Dermal (Rat): NOEL =100 mg/kg/day. The LOEL was 500
mg/kg/day based on minimal hepatocellular
vacuolation in females.
-- 90-day Oral (Rat): NOEL (systemic) =100 ppm (6.60 mg /kg/day
and 7.99 mg/kg/day for males and females, respectively). The LOEL
was 1000 ppm (68 - 79 mg/kg/day in males and females, respectively)
based on increased liver, thyroid
and kidney weights,
changes in clinical
chemistry and hematological parameters
and on gross and microscopic changes observed
in the liver and thyroid at
does levels of 68 mg/kg/day and higher. At the 201 mg/kg/day dose
diffused thyroid follicular cell hypertrophy/
hyperplasia was observed.
-- 90-day Oral (Dog): NOEL (systemic) =10 ppm. (0.2 mg/kg/day(m);
0.3 mg/kg/day(f)), based on decreased body
weight gain and increased SGPT levels at 3 and 6 m/kg/day
for males and females, respectively and above; decreased total
protein and albumin concentration and albumin/globulin ratio,
increased AP, hepatocytic hypertrophy,
oval cell proliferation and increased hepatocytic
fatty content at 35 mg/kg/day and above; and decreased
calcium concentration which is thought to be related to hypoalbuminemia,
decreased cholesterol and triglyceride concentrations, slightly
increased GGT and SGPT, follicular hyperplasia of thyroid, increased
colloid content in follicles and increased relative thyroid weight
at 175 mg/kg/day.
-- A 1 year feeding study in dogs at 0, 0.8, 7.8, 86.0 with males,
and 0.8, 8.8, and 78.0 with females with a NOEL of 0.8 mg/kg/day.
The Loel was based on hepatocellular hypertrophy
and hyperplasia. A 10% increase in prothrombin time and
\several changes in blood chemistry: increased SGOT,
SGPT, GGT and ALK levels and decreased
cholesterol, albumin and total protein and calcium were
observed in high- dose dogs. There were increases in absolute
weights, liver and body weight and
liver to brain weight, heptotoxicity
characterized by enlargement and/or discoloration in some high
dose animals and by hepatocellular hypertrophy/hyperplasia
in the 0.8 and 7.8 mg/kg/day dogs. The NOEL was based on hepatocellular
hypertrophy and hyperplasia.
-- A two-generation reproductive in rats at 0, 0.75, 7.5 and 75.0
mg/kg/day with a parental toxicity NOEL of 7.5 mg/kg/day. The
toxic effects were increased absolute and relative liver
weight, hepatic discoloration, histologic evidence of hepatic
hypertrophy and vacuolization in females in both generations.
No adverse efects were observed in adults or their offspring up
to 75 mg/kg/day, the highest dose tested.
-- A mouse carcinogenicity study at doses of 0, 0.17, 1.6, 16.9,
66.3 or 128.4 mg/kg/day (males) and 0,0.24, 2.6, 26.8, 108.1 or
215.9 mg/kg/day (female) with a systemic NOEL of 0.1 mg/kg/day.
The effects were hepatocellular hypertropy
and amyloid deposition.
At 66.3 mg/kg/day the same lesions
plus increased liver weights, random
and periportal hepatocellular vacuolation
were observed. At 128.4 mg/kg/day the
same lesions plus distended abdonen, slight increase
in ALP, SGOT and SGPT, abnormal coloration
and enlargement of liver, decrease
in absolute and relative spleen weights,
increase in absolute and relative kidney
weights, increase in eosinophilia
in hepatocytes, kidney nephropathy and lymphocytic hyperplasia
of the nesenteric lymph nodes were observed. There was no evidence
of oncoenicity at any dose level.
-- A two year rat carcinogenicity study at doses of 0, 0.04, 4.4,
44.2 or 136.4 mg/kg/day (Males) 0, 0.06, 0.6, 5.6, 56.3 or 177.1
mg/kg/day (female) with a NOEL of 4.4 mg/kg/day. The effects were
protruding eyes, evidence of mild anemia, increased GGT and cholesterol,
increased absolute and relative liver, kidney
and thyroid weights and significant
increase in microscopic lesions in the liver (hypertrophy and
vacuolar changes), kidney
(nephropathy) and thyroid (hypertrophy and hyperplasia);
decreased mean body weight and body weight gain and food consumption.
A statistically significant increase in thyroid follicular cell
adenomas/cystadenomas were observed in males at 44.2 and 136.4
mg/kg/day. A nonsignificant
increase in renal tubular adenomas in high-dose females was considered
to be equivocal.
-- The EPA Health Effects Division Carcinogenicity Peer Review
Committee classified thiazopyr as a Group
C, possible human carcinogen and recommended that for the
purpose of risk characterization a Margin of Exposure (M.O.E.)
approach should be used in evaluation of the consequences of human
exposure.
-- A second study on the effects of thiazopyr on the biochemical
mechanisms of thyroid toxicity in rats at doses of 0, 0.5, 1.5,
5, 15, 50 or 150 mg/kg/day was conducted. Dose response effects
on various biochemical parameters were observed. Two groups of
the rats in the study were observed for reversibility of effects
observed up to 56 and 112 days. Doses at 15, 50 and 150 mg/kg/day
significantly increased the liver weights.
Thyroid weights were increased at doses
of 50 and 150 mg/kg/day. There were no significant effect on body
weight or body weight gains during the study. The T4 UDPGT levels
were increased by 117 and 376% above controls at the 50 and 150
mg/kg/day dosages. Effects of 150 mg/kg/day were increases in
T3, TSH and rT3 serum concentrations, and increased incidence
of follicular cell hypertrophy/hyperplasia at the 150 mg/kg/day
dose. A NOEL of 1.5 mg/kg/day was determined based on liver weight
increases. Thyroid weight was the only parameter that did not
return to those similar to the controls. At the 56 and 112 day
recovery periods the thyroid weights were 120 and 123% of control
values, respectively.
Ref: US EPA. Pesticide Fact Sheet. Thiazopyr
Reason for Issuance: Registration of a New Chemical Date Issued:
February 20, l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
| Environmental
(click
on for all fluorinated pesticides)
Freshwater
Fish: moderately toxic
-- Rainbow trout: LC50 = 3.4 mg/L
-- Bluegill Sunfish: LC50 = 3.5 mg/L
-- Aquatic Invertebrate: moderately toxic Daphnia magna:
LC50 = 6.1 mg/L
-- Mollusc Shell Deposition: highly
toxic Eastern Oyster: EC50 = 0.82 mg/L
-- Estuarine Invertebrate Acute Toxicity: Moderately toxic
Mysid Shrimp:LC50 = 2.0 mg/L Fish Early Life Stage Toxicity
Rainbow trout: NOEL = 0.55 mg/L MATC = 0.74 mg/L
-- Aquatic Invertebrate Life Cycle Toxicity Daphnia magna:
NOEL = 0.11 mg/L MATC = 0.16 mg/L Aquatic Plant Growth and
Reproduction Selenastrum capricornutun:EC50 = 0,043 mg/L
NOEL = 0.018 mg/L
-- Thiazopyr had low toxicity to birds, mammals, honeybees,
and earthworms. It was moderately toxic to freshwater and
marine fish and Daphnia magna, with
moderate to high toxicity to marine invertebrates.
Thiazopyr was highly to very highly
toxic to nontarget terrestrial and aquatic plants, algae
and diatoms.
--
Photodegradation on soil: Thiazopyr
degrades very slowly in soil, with an extrapolated half
life of 1373 days.
Ref:
US EPA. Pesticide Fact Sheet. Thiazopyr Reason for Issuance:
Registration of a New Chemical Date Issued: February 20,
l997.
http://www.epa.gov/opprd001/factsheets/thiazopyr.pdf
|
| A
February
17, 2005,
check at the Code
of Federal Regulations for Thiazopyr (and its metabolites):
this herbicide is permitted in
or on 2 food
commodities in the United States.
The
following list identifies these crops for which EPA has set
pesticide tolerances. |
| [Code
of Federal Regulations]
[Title 40, Volume 22]
[Revised as of July 1, 2004]
From the U.S. Government Printing Office via GPO Access
[CITE: 40CFR180.496]
[Page 480]
TITLE 40--PROTECTION OF ENVIRONMENT
CHAPTER I--ENVIRONMENTAL PROTECTION AGENCY (CONTINUED)
PART 180_TOLERANCES AND EXEMPTIONS FROM TOLERANCES FOR PESTICIDE
CHEMICALS
IN FOOD--Table of Contents
Subpart C_Specific Tolerances
Sec. 180.496 Thiazopyr; tolerances for residues.
Tolerances are established for combined
residues of the herbicide
thiazopyr (3-pyridinecaroxylic acid, 2-(difluoromethyl)-5-(4,5-dihydro-
2-thiazolyl)-4-(2-methylpropyl)-6-(trifluoromethyl)-, methyl
ester) and
its metabolites determined as 2-(difluoromethyl)-6-(trifluoromethyl)-
3,4,5-pyridinetricarboxylic acid, all expressed as the parent
equivalents in or on the following raw agricultural commodities: |
| Commodities
|
Parts
per million |
Grapefruit
|
0.05 |
| Orange,
sweet |
0.05 |
|

