|
Return
to Prosulfuron Index Page
Activity:
Herbicide
(sulfonylurea)
Structure:
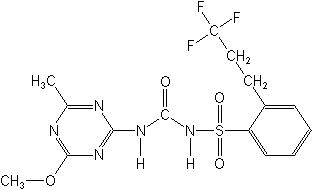
Adverse Effects:
Blood
Body Weight Decrease
Bone
Endocrine: Breast
Endocrine: Testicular
Endocrine: Uterus
Heart
Liver
•
Over the years the US EPA has granted temporary pesticide
tolerances for resiudes of Prosulfuron on raw agricultural
commodities: corn (forage, fodder, grain and fresh [including
sweet kernels], milk, and meat, fat and meat by-products,
of cattle, goats, hogs, horses, and sheep.
•
Syngenta petitioned the US EPA in December 2002 for permanent
residue tolerances for these same food groups. See petition
in Federal Register at
http://www.fluorideaction.org/pesticides/prosulfuron.fr.dec.31.2002.htm
•
See toxicological studies submitted to US EPA by Ciba-Geigy
at bottom of page
|
Blood
(click on for all fluorinated pesticides)
-- Short term toxicity
Target / critical effect: Liver (hepatocyte
hypertrophy), heart (myocardial degeneration),
hematopoietic system (red blood cells
decreased) Lowest relevant oral NOAEL / NOEL: NOAEL = 6
mg/kg b.w./day (90-day, dog)
Ref: July 2, 2002 - Review report for the
active substance prosulfuron. Finalised in the Standing Committee
on the Food Chain and Animal Health at its meeting on 26 February
2002 in view of the inclusion of prosulfuron in Annex I of Directive
91/414/EEC.European Commission Health & Consumer Protection
Directorate-General.
http://www.fluoridealert.org/pesticides/Prosulfuron.EU.July.2002.pdf
Chronic toxicity. In
the 1-year dog chronic dosing study,
the NOAEL was 1.84 mg/kg/day based on hematologic and clinical
chemistry effects and incidence of lipofuscin
accumulation in the liver at 18.6 mg/kg/day.
Ref: Federal Register: December 31, 2002.
Prosulfuron; Notice of Filing Pesticide Petitions to Establish
Tolerances for a Certain Pesticide Chemical in or on Food.
http://www.fluorideaction.org/pesticides/prosulfuron.fr.dec.31.2002.htm
-- A 2-year chronic
feeding/carcinogenicity study in rats
fed dosages of 0, 0.4, 7.9, 79.9 or 160.9 (males), and 0, 0.5,
9.2, 95.7 or 205.8 mg/kg/day (females) was conducted. There was
uncertain evidence of carcinogenicity with slight increases in
the incidence of mammary gland adenocarcinomas
in females at 95.7 and 205.8 mg/kg/day, slight increase in incidence
of benign testicular interstitial cell tumors at 79.9 and 160.9
mg/kg/day (significant trend only). A systemic NOAEL of
7.9 mg/kg/day was based on decreased body
weight and body weight gain, hematopoietic
effects (males), and possibly increased
serum GGT and decreased liver, kidney and adrenal weights
(females) at 79.9 mg/kg/ day.
Ref: Federal Register: August 25, 1999.
[PF-882; FRL-6093-7]. Notice of Filing a Pesticide Petition to
Establish a Tolerance for Certain Pesticide Chemicals in or on
Food.
http://www.fluorideaction.org/pesticides/prosulfuron.fr.aug.25.1999.htm
Body
Weight Decrease (click
on for all fluorinated pesticides)
-- A 2-year chronic
feeding/carcinogenicity study in rats fed dosages of 0, 0.4, 7.9,
79.9 or 160.9 (males), and 0, 0.5, 9.2, 95.7 or 205.8 mg/kg/day
(females) was conducted. There was uncertain evidence of carcinogenicity
with slight increases in the incidence of
mammary gland adenocarcinomas in females at 95.7 and 205.8 mg/kg/day,
slight increase in incidence of benign testicular interstitial
cell tumors at 79.9 and 160.9 mg/kg/day (significant trend only).
A systemic NOAEL of 7.9 mg/kg/day was based on
decreased body weight and body weight gain, hematopoietic
effects (males), and possibly increased serum GGT and decreased
liver, kidney and adrenal weights (females) at 79.9 mg/kg/
day.
Ref: Federal Register: August 25, 1999.
[PF-882; FRL-6093-7]. Notice of Filing a Pesticide Petition to
Establish a Tolerance for Certain Pesticide Chemicals in or on
Food.
http://www.fluorideaction.org/pesticides/prosulfuron.fr.aug.25.1999.htm
Bone
(click
on for all fluorinated pesticides)
-- Reproductive toxicity.
Developmental toxicity: Skeletal variations
(rat) and resorptions (rabbit) at maternal toxic doses.
Lowest relevant developmental NOAEL / NOEL: NOAEL = 10 mg/kg b.w./day
(rabbit)
Ref: July 2, 2002 - Review
report for the active substance prosulfuron. European Commission
Health & Consumer Protection Directorate-General.
Endocrine:
Breast (click
on for all fluorinated pesticides)
-- Long term toxicity and carcinogenicity Target / critical effect:
Liver (hepatocellular hypertrophy in mice), indication
of hormonal disruption (uterus and
mammalian gland in rats) at high dose levels. Lowest relevant
NOAEL: NOAEL = 1,7 mg/kg bw/day (18-month, mouse) Carcinogenicity:
No carcinogenic potential
Ref: July 2, 2002 - Review
report for the active substance prosulfuron. European Commission
Health & Consumer Protection Directorate-General.
-- A 2-year chronic feeding/carcinogenicity study in rats fed
dosages of 0, 0.4, 7.9, 79.9 or 160.9 (males), and 0, 0.5, 9.2,
95.7 or 205.8 mg/kg/day (females) was conducted. There was uncertain
evidence of carcinogenicity with slight increases in the incidence
of mammary gland adenocarcinomas in
females at 95.7 and 205.8 mg/kg/day, slight increase in incidence
of benign testicular interstitial cell tumors
at 79.9 and 160.9 mg/kg/day (significant trend only). A systemic
NOAEL of 7.9 mg/kg/day was based on decreased body weight and
body weight gain, hematopoietic effects (males), and possibly
increased serum GGT and decreased liver, kidney and adrenal weights
(females) at 79.9 mg/kg/ day.
-- Carcinogenicity. The HED RfD/Peer Review Committee (PRC) classified
this chemical as a Class D oncogen based on the conclusion that
there was uncertain evidence of carcinogenicity. No relevant treatment-related
oncogenic potential was observed in rats and mice. The slightly
increased incidence of testicular interstitial
cell tumors in male rats at 79.9 mg/kg/day and the
slightly increased incidence of mammary
gland adenocarcinoma in females at 95.7 mg/kg/day
was not significant when the increased survival in these groups
was considered. Furthermore, the overall incidence of mammary
tumors was essentially identical in all groups including the control.
There was no evidence of carcinogenicity in mice and dosages in
both studies were sufficient for identifying a cancer risk. In
the absence of carcinogenicity, it is appropriate that a RfD approach
be used for quantitation of human risks.
Ref: Federal Register: August 25, 1999.
[PF-882; FRL-6093-7]. Notice of Filing a Pesticide Petition to
Establish a Tolerance for Certain Pesticide Chemicals in or on
Food.
http://www.fluorideaction.org/pesticides/prosulfuron.fr.aug.25.1999.htm
Endocrine:
Testicular (click
on for all fluorinated pesticides)
-- A 2-year chronic
feeding/carcinogenicity study in rats fed dosages of 0, 0.4, 7.9,
79.9 or 160.9 (males), and 0, 0.5, 9.2, 95.7 or 205.8 mg/kg/day
(females) was conducted. There was uncertain evidence of carcinogenicity
with slight increases in the incidence of mammary
gland adenocarcinomas in females at 95.7 and 205.8 mg/kg/day,
slight increase in incidence of benign testicular
interstitial cell tumors at 79.9 and 160.9 mg/kg/day (significant
trend only). A systemic NOAEL of 7.9 mg/kg/day was based
on decreased body weight and body weight
gain, hematopoietic effects (males), and possibly increased serum
GGT and decreased liver, kidney and adrenal weights (females)
at 79.9 mg/kg/ day.
-- Carcinogenicity. The HED RfD/Peer Review Committee (PRC) classified
this chemical as a Class D oncogen based on the conclusion that
there was uncertain evidence of carcinogenicity. No relevant treatment-related
oncogenic potential was observed in rats and mice. The slightly
increased incidence of testicular interstitial
cell tumors in male rats at 79.9 mg/kg/day
and the slightly increased incidence of
mammary gland adenocarcinoma in females at
95.7 mg/kg/day was not significant when the increased survival
in these groups was considered. Furthermore, the overall incidence
of mammary tumors was essentially identical in all groups including
the control. There was no evidence of carcinogenicity in mice
and dosages in both studies were sufficient for identifying a
cancer risk. In the absence of carcinogenicity, it is appropriate
that a RfD approach be used for quantitation of human risks.
Ref: Federal Register: August 25, 1999.
[PF-882; FRL-6093-7]. Notice of Filing a Pesticide Petition to
Establish a Tolerance for Certain Pesticide Chemicals in or on
Food.
http://www.fluorideaction.org/pesticides/prosulfuron.fr.aug.25.1999.htm
Endocrine:
Uterus (click
on for all fluorinated pesticides)
-- Long term toxicity
and carcinogenicity Target / critical effect:
Liver (hepatocellular hypertrophy in mice), indication
of hormonal disruption (uterus and mammalian
gland in rats) at high dose levels. Lowest relevant
NOAEL: NOAEL = 1,7 mg/kg bw/day (18-month, mouse) Carcinogenicity:
No carcinogenic potential
Ref: July 2, 2002 - Review
report for the active substance prosulfuron. European Commission
Health & Consumer Protection Directorate-General.
Heart
(click
on for all fluorinated pesticides)
-- Short term toxicity
Target / critical effect: Liver (hepatocyte
hypertrophy), heart (myocardial degeneration),
hematopoietic system (red blood cells decreased) Lowest relevant
oral NOAEL / NOEL: NOAEL = 6 mg/kg b.w./day (90-day, dog)
Ref: July 2, 2002 - Review report for the
active substance prosulfuron. Finalised in the Standing Committee
on the Food Chain and Animal Health at its meeting on 26 February
2002 in view of the inclusion of prosulfuron in Annex I of Directive
91/414/EEC.European Commission Health & Consumer Protection
Directorate-General.
http://www.fluoridealert.org/pesticides/Prosulfuron.EU.July.2002.pdf
Liver
(click
on for all fluorinated pesticides)
-- Short term toxicity
Target / critical effect: Liver (hepatocyte
hypertrophy), heart (myocardial degeneration), hematopoietic
system (red blood cells decreased) Lowest relevant oral NOAEL
/ NOEL: NOAEL = 6 mg/kg b.w./day (90-day, dog)
-- Long term toxicity and carcinogenicity Target / critical effect:
Liver (hepatocellular hypertrophy in mice), indication
of hormonal disruption (uterus and mammalian gland in rats) at
high dose levels Lowest relevant NOAEL: NOAEL = 1,7 mg/kg bw/day
(18-month, mouse) Carcinogenicity: No carcinogenic potential
Ref: July 2, 2002 - Review report for the
active substance prosulfuron. European Commission Health &
Consumer Protection Directorate-General.
http://www.fluoridealert.org/pesticides/Prosulfuron.EU.July.2002.pdf
-- In an 18-month mouse
carcinogenicity study, prosulfuron was administered at dose levels
of 0, 1.71, 81.4, 410 or 832 mg/kg/day (males), and 0, 2.11, 100,
508 or 1,062 mg/kg/day (females). There was no evidence of carcinogenic
effects up to 1,062 mg/kg/day, the HDT. The NOAEL was 1.71 mg/kg/day
in males, and 100 mg/kg/day in females based on increased incidence/severity
of centrilobular hepatocellular hypertrophy.
-- Chronic toxicity. Prosulfuron was fed to dogs at dosages of
0, 0.33, 1.95, 18.6 or 41.0 mg/kg/day (males) and 0, 0.31, 1.84,
20.2, or 48.8 mg/kg/day (females) for 1-year. The NOAEL was 1.84
mg/kg/day based on hematologic and clinical chemistry effects
and incidence of lipofuscin accumulation
in the liver at 18.6 mg/kg/day.
Ref: Federal Register: August 25, 1999.
[PF-882; FRL-6093-7]. Notice of Filing a Pesticide Petition to
Establish a Tolerance for Certain Pesticide Chemicals in or on
Food.
http://www.fluorideaction.org/pesticides/prosulfuron.fr.aug.25.1999.htm
|
Environmental
(click
on for all fluorinated pesticides)
Particular
conditions to be taken into account on short term basis
by Member States in relation to the granting of authorisations
of plant protection products containing prosulfuron On the
basis of the proposed and supported uses [herbicide
on maize, sorghum and sweet corn], the following
particular issues have been identified as requiring particular
and short term (within 12 months at the latest) attention
from the Member States, in the framework of any authorisations
to be granted, varied or withdrawn, as appropriate:
- Aquatic
organisms: Member states must carefully
consider the risk to aquatic plants if the active substance
is applied adjacent to surface waters. The exposure
input from drain flow with respect to local conditions should
also be considered. Risk mitigation measures (e.g. buffer
zones) should be applied where appropriate.
- Leaching
to groundwater: Particular attention should be given to
the potential for groundwater contamination, when the active
substance is applied in regions with vulnerable soil and/or
climate conditions. Risk mitigation measures should
be applied where appropriate.
Ref:
July 2, 2002 - Review report for the active substance prosulfuron.
European Commission Health & Consumer Protection Directorate-General.
http://www.fluoridealert.org/pesticides/Prosulfuron.EU.July.2002.pdf
|
Ref:
Toxline at Toxnet
as of October 14, 2003.
Note: If anyone has copies of the
following studies would they please share them with FAN's
Pesticide Project. Thanks- EC.
|
| SUPPORT:
LETTER FROM CIBA GEIGY CORP TO USEPA REGARDING TOXICOLOGICAL
STUDIES OF CGA-152005 WITH ATTACHMENTS DATED
02-02-95
Source:
EPA/OTS; Doc #89-950000124
Keywords:
CIBA GEIGY CORP
CGA-152005
HEALTH EFFECTS
SUBCHRONIC TOXICITY
MAMMALS
DOGS
ORAL
DIET
REPRODUCTION/FERTILITY EFFECTS
TERATOGENICITY
RABBITS
GAVAGE
MICE
NEUROTOXICITY
RATS
CAS No:
94125-34-5
Order
Number:
NTIS/OTS0529902-3 |
| SUPPORT:
LETTER FROM CIBA GEIGY CORP TO USEPA RE: TWO-YEAR DIETARY
CHRONIC TOXICITY/ONCOGENICITY STUDY WITH CGA-152005 TECHNICAL
IN RATS WITH ATTACHMENTS DATED
04-08-94
Source:
EPA/OTS; Doc #8EHQ-0494-1104
Keywords:
CIBA
GEIGY CORP
CGA-152005
HEALTH EFFECTS
CHRONIC TOXICITY
CARCINOGENICITY
MAMMALS
RATS
ORAL
DIET
CAS No:
94125-34-5
Order
Number:
NTIS/OTS0529902-3 |
| SUPPORT:
LETTER FROM CIBA-GEIGY CORP TO USEPA RE: 1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPROPYL)PHENYLSULFONYL]UREA,
DATED
09/21/1992 (DECLASSIFIED)
Source:
EPA/OTS; Doc #89920000360D
Keywords:
CIBA-GEIGY CORP
1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPRO*
HEALTH EFFECTS
CHRONIC TOXICITY
COMBINED CHRONIC TOXICITY/CARCINOGENICITY
MAMMALS
RATS
ORAL
DIET
CAS No:
94125-34-5
Order
Number:
NTIS/OTS0529902-4 |
| SUPPORT:
LETTER FROM CIBA-GEIGY CORP TO USEPA RE: 1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPROPYL)PHENYLSULFONYL]UREA,
DATED
05/30/1991 (DECLASSIFIED)
Source:
EPA/OTS; Doc #89910000154D
Keywords:
CIBA-GEIGY CORP
1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPRO*
HEALTH EFFECTS
SUBCHRONIC TOXICITY
MAMMALS
MICE
ORAL
DIET
CAS No:
94125-34-5
Order
Number: NTIS/OTS0529902-4
|
| SUPPORT:
LETTER FROM CIBA-GEIGY CORP TO USEPA RE: 1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPROPYL)PHENYLSULFONYL]UREA,
DATED
01/02/1991 (DECLASSIFIED)
Source:
EPA/OTS; Doc #89910000026D
Keywords:
CIBA-GEIGY CORP
1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPRO*
HEALTH EFFECTS
REPRODUCTION/FERTILITY EFFECTS
TERATOGENICITY
MAMMALS
RABBITS
ORAL CAS
CAS No:
94125-34-5
Order
Number: NTIS/OTS0529902-4 |
| SUPPORT:
LETTER FROM CIBA-GEIGY CORP TO USEPA RE: 1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPROPYL)PHENYLSUFONYL]UREA,
DATED
09/03/1991 (DECLASSIFIED)
Source:
EPA/OTS; Doc #89910000273D
Keywords:
CIBA-GEIGY CORP
1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPRO*
HEALTH EFFECTS
SUBCHRONIC TOXICITY
MAMMALS
DOGS
ORAL
DIET
CAS
No: 94125-34-5
Order
Number: NTIS/OTS0529902-4 |
| INITIAL
SUBMISSION: LETTER FROM CIBA-GEIGY CORP TO USEPA RE: 1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPROPYL)PHENYLSULFONYL]UREA,
DATED 10/19/1990
(DECLASSIFIED)
Source:
EPA/OTS; Doc #88910000017D
Keywords:
CIBA-GEIGY CORP
1-(4-METHOXY-6-METHYLTRIAZIN-2-YL)-3-[2-(3,3,3-TRIFLUOROPRO*
HEALTH EFFECTS
REPRODUCTION/FERTILITY EFFECTS
TERATOGENICITY
MAMMALS
RABBITS
ORAL
SUBCHRONIC TOXICITY
DOGS
DIET
CAS No:
94125-34-5
Order
Number:
NTIS/OTS0529902-4 |
| Ref:
Toxline at Toxnet
as of October 14, 2003. |
|

