|
Return
to Index Page
Activity:
Fungicide,
Herbicide (sulfonylurea)
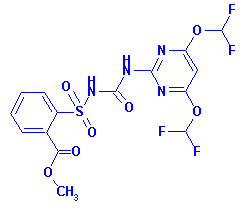
Structure:
Adverse
Effects:
Anemia
Blood
Body
Weight Decrease
Bone
Endocrine: Testicular
Endocrine: Thyroid
Kidney
Liver
Spleen
Environmental
As
of February 15, 2005, this herbicide is permitted in
or on 24 food commodities
in the United States - see list at bottom of page.
|
Anemia
(click on for all fluorinated
pesticides)
In a 90-day dog feeding
study, reduced thyroid weights accompanied by colloid depletion
and parafollicular hyperplasia and anemia were observed at the
LOEL of 25 mg/kg/day. The NOEL was 0.625 mg/kg/day. In a 1-year
dog study, dietary administration of 250/125 mg/kg/day (LOEL:
the dose was changed after week 10 in the study) produced thyroid
hyperplasia, anemia, increased
platelet levels, vacuolar changes, and increased absolute
and relative liver weights. The NOEL was 25 mg/kg/day. In an 18-month
study in mice, dietary administration of 1.7 mg/kg/day produced
increased absolute and relative liver weights in females. No NOEL
was established...
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
Blood
(click on for
all fluorinated pesticides)
In a 90-day dog feeding
study, reduced thyroid weights accompanied by colloid depletion
and parafollicular hyperplasia and anemia were observed at the
LOEL of 25 mg/kg/day. The NOEL was 0.625 mg/kg/day. In a 1-year
dog study, dietary administration of 250/125 mg/kg/day (LOEL:
the dose was changed after week 10 in the study) produced thyroid
hyperplasia, anemia, increased
platelet levels, vacuolar changes, and increased absolute
and relative liver weights. The NOEL was 25 mg/kg/day. In an 18-month
study in mice, dietary administration of 1.7 mg/kg/day produced
increased absolute and relative liver weights in females. No NOEL
was established...
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
Body
Weight Decrease (click
on for all fluorinated pesticides)
-- Chronic toxicity:
Doses of 125 mg/kg/day administered in the diet to dogs over a
1-year period produced decreased body weight
gain, anemia, increased liver weight,
and thyroid hyperplasia (abnormal growth) [15]. Rats fed dietary
doses of about 180 mg/kg/day over 90 days showed effects similar
to those noted in dogs, as well as spleen weight increases [24].
In another study, doses of 480 mg/kg/day in rats over 18 months
produced increased incidence of tooth disorders, chronic nephritis
(kidney damage), and testicular atrophy [4]. In two 18-month studies
in mice, testicular atrophy, chronic nephritis, and increased
tooth and bone disorders were
seen at doses of 180 mg/kg/day and 360 mg/kg/day, respectively
[4].
-- Reproductive effects: Changes in the
function of the testes were noted in rats fed high doses (250
mg/kg/day) of primisulfuron-methyl over two generations. There
was also a decrease in the body weight of
the offspring. No compound-related effects on reproduction
were noted at doses below 50 mg/kg/day [15]. Testicular atrophy
was seen in rats in chronic studies (see above), which
could result in reproductive effects. The available data suggest
that reproductive effects in humans due to primisulfuron are not
likely under normal circumstances.
Ref: E X T O X N E T Extension Toxicology
Network Pesticide Information Profiles.
Revised June 1996.
http://ace.ace.orst.edu/info/extoxnet/pips/primisul.htm
Bone
(click on for all fluorinated
pesticides)
-- Chronic toxicity:
Doses of 125 mg/kg/day administered in the diet to dogs over a
1-year period produced decreased body weight gain, anemia, increased
liver weight, and thyroid hyperplasia (abnormal growth) [15].
Rats fed dietary doses of about 180 mg/kg/day over 90 days showed
effects similar to those noted in dogs, as well as spleen weight
increases [24]. In another study, doses of 480 mg/kg/day in rats
over 18 months produced increased incidence of tooth disorders,
chronic nephritis (kidney damage), and testicular atrophy [4].
In two 18-month studies in mice, testicular atrophy, chronic nephritis,
and increased tooth and bone disorders
were seen at doses of 180 mg/kg/day and 360 mg/kg/day, respectively
[4].
-- Teratogenic effects: No teratological effects were seen in
offspring of rabbits given doses of up to 600 mg/kg/day. In one
study of rats, delayed skeletal development
and lack of ossification was seen in offspring of pregnant
rats given doses of 500 mg/kg/day, while in another, 100 mg/kg/day
produced incomplete ossification of the
pubic bone [15]. The available evidence suggests that primisulfuron-methyl
is not teratogenic except at very high doses.
-- Organ toxicity: Target organs identified in animal studies
include the liver, kidneys, spleen, and
testes, as well as the skeleton.
Ref: E X T O X N E T Extension Toxicology
Network Pesticide Information Profiles. Revised June 1996.
http://ace.ace.orst.edu/info/extoxnet/pips/primisul.htm
-- Acute toxicity.
For acute dietary risk assessment, the Agency used a NOEL of 100
mg/kg/day, based on delayed or absent ossification
effects in fetuses at the LEL of 500 mg/kg/day, from the oral
developmental study in rats. This risk assessment will evaluate
acute dietary risk to the population subgroup of concern, females
13+ years of age... Acute risk. The finding of developmental effects
in the rat study absent ossification
required that an acute dietary risk assessment be performed for
females 13+ years of age. The calculated MOE of 71,000 demonstrated
that acute pre-natal developmental risks were below EPA's level
of concern.
Ref: Federal Register: December 17, 1997.Primisulfuron-methyl;
Pesticide Tolerances for Emergency Exemptions. Final Rule.
http://www.fluoridealert.org/pesticides/Primisulfuron-methyl.FR.97.htm
Endocrine:
Testicular (click
on for all fluorinated pesticides)
-- Chronic toxicity:
Doses of 125 mg/kg/day administered in the diet to dogs over a
1-year period produced decreased body weight gain, anemia, increased
liver weight, and thyroid hyperplasia
(abnormal growth) [15]. Rats fed dietary doses of about
180 mg/kg/day over 90 days showed effects similar to those noted
in dogs, as well as spleen weight increases
[24]. In another study, doses of 480 mg/kg/day in rats over 18
months produced increased incidence of tooth disorders, chronic
nephritis (kidney damage), and testicular
atrophy [4].
In two 18-month studies in mice, testicular
atrophy, chronic nephritis, and increased
tooth and bone disorders were seen at doses of 180 mg/kg/day
and 360 mg/kg/day, respectively [4].
-- Reproductive effects: Changes in the function of the
testes were noted in rats fed high doses (250 mg/kg/day)
of primisulfuron-methyl over two generations. There was also a
decrease in the body weight of the offspring. No compound-related
effects on reproduction were noted at doses below 50 mg/kg/day
[15]. Testicular atrophy was seen
in rats in chronic studies (see above), which could result in
reproductive effects. The available data suggest that reproductive
effects in humans due to primisulfuron are not likely under normal
circumstances.
-- Organ toxicity: Target organs identified in animal studies
include the liver, kidneys, spleen, and
testes, as well
as the skeleton.
Ref: E X T O X N E T Extension Toxicology
Network Pesticide Information Profiles. Revised June 1996.
http://ace.ace.orst.edu/info/extoxnet/pips/primisul.htm
Endocrine:
Thyroid (click
on for all fluorinated pesticides)
-- Chronic toxicity:
Doses of 125 mg/kg/day administered in the diet to dogs over a
1-year period produced decreased body weight gain, anemia, increased
liver weight, and thyroid hyperplasia
(abnormal growth) [15]. Rats fed dietary doses of about
180 mg/kg/day over 90 days showed effects similar to those noted
in dogs, as well as spleen weight increases
[24]. In another study, doses of 480 mg/kg/day in rats over 18
months produced increased incidence of tooth disorders, chronic
nephritis (kidney damage), and testicular
atrophy [4]. In two 18-month studies in mice, testicular atrophy,
chronic nephritis, and increased tooth and bone disorders were
seen at doses of 180 mg/kg/day and 360 mg/kg/day, respectively
[4].
Ref: E X T O X N E T Extension Toxicology
Network Pesticide Information Profiles. Revised June 1996.
http://ace.ace.orst.edu/info/extoxnet/pips/primisul.htm
In a 90-day dog feeding
study, reduced thyroid weights accompanied
by colloid depletion and parafollicular hyperplasia and anemia
were observed at the LOEL of 25 mg/kg/day. The NOEL was 0.625
mg/kg/day. In a 1-year dog study, dietary administration of 250/125
mg/kg/day (LOEL: the dose was changed after week 10 in the study)
produced thyroid hyperplasia, anemia,
increased platelet levels, vacuolar changes, and increased absolute
and relative liver weights. The NOEL was 25 mg/kg/day. In an 18-month
study in mice, dietary administration of 1.7 mg/kg/day produced
increased absolute and relative liver weights in females. No NOEL
was established. Based on this study, an oral RfD of 0.006 mg/kg/day
was derived. In a 2-year mouse study, increases in absolute and
relative liver weights were observed at 408 mg/kg/day in males
and 1.7 mg/kg/day in females. The systemic LOEL and NOEL in males
was 408 mg/kg/day and 40.2 mg/kg/day, respectively. The systemic
LOEL in females was 1.7 mg/kg/day and a NOEL could not be established.
EPA believes that there is sufficient evidence for listing primisulfuron
on EPCRA section 313 pursuant to EPCRA section 313(d)(2)(B) based
on the available thyroid and liver
toxicity data for this chemical.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
Kidney
(click on for all fluorinated
pesticides)
-- Chronic toxicity:
Doses of 125 mg/kg/day administered in the diet to dogs over a
1-year period produced decreased body weight gain, anemia, increased
liver weight, and thyroid hyperplasia (abnormal growth) [15].
Rats fed dietary doses of about 180 mg/kg/day over 90 days showed
effects similar to those noted in dogs, as well as spleen weight
increases [24]. In another study, doses of 480 mg/kg/day in rats
over 18 months produced increased incidence of tooth disorders,
chronic nephritis (kidney damage),
and testicular atrophy [4]. In two 18-month studies in mice, testicular
atrophy, chronic nephritis, and increased tooth and bone disorders
were seen at doses of 180 mg/kg/day and 360 mg/kg/day,
respectively [4].
-- Organ toxicity: Target organs
identified in animal studies include the
liver, kidneys,
spleen, and testes,
as well as the skeleton.
Ref: E X T O X N E T Extension Toxicology
Network Pesticide Information Profiles. Revised June 1996.
http://ace.ace.orst.edu/info/extoxnet/pips/primisul.htm
Liver
(click
on for all fluorinated pesticides)
-- Chronic toxicity:
Doses of 125 mg/kg/day administered in the diet to dogs over a
1-year period produced decreased body weight gain, anemia, increased
liver weight, and thyroid hyperplasia
(abnormal growth) [15]...
-- Reproductive effects: Changes in the function of the testes
were noted in rats fed high doses (250 mg/kg/day) of primisulfuron-methyl
over two generations. There was also a decrease in the body weight
of the offspring. No compound-related effects on reproduction
were noted at doses below 50 mg/kg/day [15]. Testicular atrophy
was seen in rats in chronic studies (see above), which
could result in reproductive effects. The available data suggest
that reproductive effects in humans due to primisulfuron are not
likely under normal circumstances.
-- Organ toxicity: Target organs identified in animal studies
include the liver, kidneys,
spleen, and testes,
as well as the skeleton.
Ref: E X T O X N E T Extension Toxicology
Network Pesticide Information Profiles. Revised June 1996.
http://ace.ace.orst.edu/info/extoxnet/pips/primisul.htm
-- Chronic toxicity.
EPA has established the RfD for primisulfuron- methyl at 0.006
milligrams/kilogram/day (mg/kg/day). This RfD is based on an 80-week
mouse feeding study with a LOEL of 1.7 mg/kg/day based on increased
absolute and relative liver weights.
An uncertainty factor of 300 was used because a NOEL was not achieved.Carcinogenicity.
Primisulfuron-methyl has been classified as a Group D chemical
incomplete evidence based on liver
tumors in mice.
Ref: Federal Register: December 17, 1997.Primisulfuron-methyl;
Pesticide Tolerances for Emergency Exemptions. Final Rule.
http://www.fluoridealert.org/pesticides/Primisulfuron-methyl.FR.97.htm
In a 1-year dog study, dietary administration of 250/125 mg/kg/day
(LOEL: the dose was changed after week 10 in the study) produced
thyroid hyperplasia, anemia, increased platelet levels, vacuolar
changes, and increased absolute and relative liver
weights. The NOEL was 25 mg/kg/day. In an 18-month study in mice,
dietary administration of 1.7 mg/kg/day produced increased absolute
and relative liver weights in females.
No NOEL was established. Based on this study, an oral RfD of 0.006
mg/kg/day was derived. In a 2-year mouse study, increases in absolute
and relative liver weights were observed
at 408 mg/kg/day in males and 1.7 mg/kg/day in females. The systemic
LOEL and NOEL in males was 408 mg/kg/day and 40.2 mg/kg/day, respectively.
The systemic LOEL in females was 1.7 mg/kg/day
and a NOEL could not be established. EPA believes that there is
sufficient evidence for listing primisulfuron on EPCRA section
313 pursuant to EPCRA section 313(d)(2)(B) based on the available
thyroid and liver toxicity data for
this chemical.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
Spleen
(click on for all fluorinated pesticides)
Organ toxicity: Target
organs identified in animal studies include
the liver, kidneys, spleen,
and testes,
as well as the skeleton.
Ref: E X T O X N E T Extension Toxicology
Network Pesticide Information Profiles. Revised June 1996.
http://ace.ace.orst.edu/info/extoxnet/pips/primisul.htm
| Environmental
(click
on for all fluorinated pesticides)
Plant
toxicity values include a duckweed 14-day EC
50 of 0.27 ppb and an algae
7-day EC 50 of 24 ppb. EPA believes that there
is sufficient evidence for listing primisulfuron on EPCRA
section 313 pursuant to EPCRA section 313(d)(2)(C) based
on the available environmental toxicity data for this chemical.
Ref: USEPA/OPP. Support
Document for the Addition of Chemicals from Federal Insecticide,
Fungicide, Rodenticide Act (FIFRA) Active Ingredients to
EPCRA Section 313. U. S. Environmental Protection Agency,
Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372.
Addition of Certain Chemicals; Toxic Chemical Release Reporting;
Community Right-to-Know; Proposed Rule.
|
A
February
15, 2005,
check at the Code
of Federal Regulations for Primisulfuron-methyl: this
herbicide is permitted in or
on 24 food
commodities in the United States.
The
following list identifies these crops for which EPA has
set pesticide tolerances.
|
| [Code
of Federal Regulations]
[Title 40, Volume 22]
[Revised as of July 1, 2004]
From the U.S. Government Printing Office via GPO Access
[CITE: 40CFR180.452]
[Page 460]
TITLE 40--PROTECTION OF ENVIRONMENT
CHAPTER I--ENVIRONMENTAL PROTECTION AGENCY (CONTINUED)
PART 180_TOLERANCES AND EXEMPTIONS FROM TOLERANCES FOR PESTICIDE
CHEMICALS
IN FOOD--Table of Contents
Subpart C_Specific Tolerances
Sec. 180.452 Primisulfuron-methyl; tolerances for residues.
(a) General. Tolerances are established for residues of
primisulfuron-methyl (3-[4,6-bis-(difluoromethoxy)-pyrimidin-2-yl]-1-(2-
methoxycarbonylphenylsulfonyl) urea) in or on the following
raw
agricultural commodities. |
| Crop |
As
of October 8, 2003
PPM |
As
of
February 15,
2005
PPM |
| Cattle,
fat |
0.1 |
0.10 |
| Cattle,
meat |
0.1 |
0.10 |
| Cattle,
meat byproducts |
0.1 |
0.10 |
| Corn, forage |
0.1 |
0.10 |
| Corn, fresh
(including sweet kernels plus cobs with husks removed) |
0.1 |
0.10 |
| Corn, grain
|
0.02 |
0.02 |
| Corn, stover |
Not listed |
0.10 |
| Egg |
0.1 |
0.10 |
| Goat, fat |
0.1 |
0.10 |
| Goat, meat |
0.1 |
0.10 |
| Goat, meat
byproducts |
0.1 |
0.10 |
| Hog, fat |
0.1 |
0.10 |
| Hog, meat |
0.1 |
0.10 |
| Hog, meat
byproducts |
0.1 |
0.10 |
| Horse,
fat |
0.1 |
0.10 |
| Horse,
meat |
0.1 |
0.10 |
| Horse,
meat byproducts |
0.1 |
0.10 |
| Milk |
0.02 |
0.02 |
| Poultry,
fat |
0.1 |
0.10 |
| Poultry,
meat |
0.1 |
0.10 |
| Poultry,
meat byproducts |
0.1 |
0.10 |
| Sheep,
fat |
0.1 |
0.10 |
| Sheep,
meat |
0.1 |
0.10 |
| Sheep,
meat byproducts |
0.1 |
0.10 |
| CATTLE,
FAT |
0.1 |
metabolites
not listed |
| CATTLE,
MEAT |
0.1 |
metabolites
not listed |
| CATTLE,
MEAT BYPRODUCTS |
0.1 |
metabolites
not listed |
| CORN,
FODDER |
0.1 |
Not
listed |
| CORN,
FODDER |
0.1 |
metabolites not listed |
| CORN,
FORAGE |
0.1 |
metabolites
not listed |
| CORN,
FRESH (INC. SWEET)(K+CWHR) |
0.1 |
metabolites
not listed |
| CORN,
GRAIN |
0.02 |
metabolites
not listed |
| EGG |
0.1 |
metabolites
not listed |
| GOAT,
FAT |
0.1 |
metabolites
not listed |
| GOAT,
MEAT |
0.1 |
metabolites
not listed |
| GOAT,
MEAT BYPRODUCTS |
0.1 |
metabolites
not listed |
| HOG, FAT |
0.1 |
metabolites
not listed |
| HOG, MEAT |
0.1 |
metabolites
not listed |
| HOG, MEAT
BYPRODUCTS |
0.1 |
metabolites
not listed |
| HORSE,
FAT |
0.1 |
metabolites
not listed |
| HORSE,
MEAT |
0.1 |
metabolites
not listed |
| HORSE,
MEAT BYPRODUCTS |
0.1 |
metabolites
not listed |
| MILK |
0.02 |
metabolites
not listed |
| POULTRY,
FAT |
0.1 |
metabolites
not listed |
| POULTRY,
MEAT |
0.1 |
metabolites
not listed |
| POULTRY,
MEAT BYPRODUCTS |
0.1 |
metabolites
not listed |
| SHEEP,
FAT |
0.1 |
metabolites
not listed |
| SHEEP,
MEAT |
0.1 |
metabolites
not listed |
| SHEEP,
MEAT BYPRODUCTS |
0.1 |
metabolites
not listed |
| (b)
Section 18 emergency exemptions. [Reserved]
(c) Tolerances with regional registrations. [Reserved]
(d) Indirect or inadvertent residues. [Reserved] |
|

