|
Return
to Novaluron Index Page
Activity:
Insecticide
(Benzoylurea)
Structure:
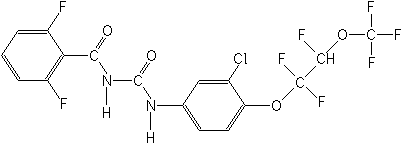
Adverse
Effects:
Anemia
Blood
Body Weight Decrease
CNS
Endocrine: Testes
Liver
Reproductive and Developmental
Spleen
ENVIRONMNETAL
NOTE:
Toxicology Summary... The main toxicological
effect noted in the animal database was oxidative stress and destruction
of red blood cells (RBCs), most likely
due to the action of an aniline metabolite (3-chloro-4-(1,1,2-trifluoromethoxy)
aniline).... (page 10)
Ref: Proposed
Registration Decision. PRD2006-05. Health Canada Pest Management
Regulatory Agency. December 22, 2006.
http://www.fluorideaction.org/pesticides/novaluron.canada.12-22-06.pdf
...
The highest level among the tissue
concentrations was found in the fat, followed by liver,
kidney, pancreas, and lymph node. Compared
with the animals treated with a Single Low Dose, the tissue
concentrations in the animals with a Single High Dose were
about 50 to 90 times higher with a 500-fold increase in
dosage. Concerning the tissue concentrations, they had a
3 to 5-fold increase in the animals with Repeated administrations,
compared with those in the animals with a Single Low Dose.
The half-lives (hereinafter “DT50”) of the radioactivity
in the fats after the treatment with the Repeated Low Dose
were 52 h in males and 56 h in females. The facts that NOVALURON
is not relatively metabolized easily, and that the unchanged
substances mainly distribute in fat tissue because of its
high LogPow (4.3), is retained there, and is
eliminated only slowly from the tissues, are assumed to
result in the high level of concentration in the fats...
[page 5]
Ref: December 2003: Evaluation
Report NOVALURON. Japan Food Safety Commission. Pesticides
Expert Committee.
|
Anemia
(click
on for all fluorinated pesticides)
From
Table 2.--Summary of Toxicological Dose and Endpoints for novaluron
for Use in Human Risk Assessment:
--
Chronic
dietary (All populations);
Long-term inhalation (>6 months) and
Long-term dermal (>6months).
Combined chronic toxicity carcinogenicity feeding in rat LOAEL
= 30.6 mg/kg/day based on erythrocyte damage and turnover resulting
in a regenerative anemia.
Re: June 2, 2004. Novaluron; Pesticide Tolerance.
Final Rule. Federal Register.
http://www.fluoridealert.org/pesticides/novaluron.fr.june.2.2004.htm
Blood
(click on for all fluorinated pesticides)
Toxicology Summary... The main toxicological effect noted in
the animal database was oxidative stress and destruction of red
blood cells (RBCs), most likely due to the
action of an aniline metabolite (3-chloro-4-(1,1,2-trifluoromethoxy)
aniline). As a result of erythrocyte destruction, secondary
effects were observed in associated blood tissues/organs and included
pigmentation in Kupffer cells in the liver as well as macrophages
in the spleen. At higher doses, the effect on red blood cell parameters
was of a sufficient magnitude to result in hemolytic anaemia and
provoke a regenerative response as evidenced by an increase in
reticulocytes, Howell-Jolly Bodies and/or Heinz bodies, with accompanying
hyperplastic response in the bone marrow and spleen... (pages
10-11)
Ref: Proposed
Registration Decision. PRD2006-05. Health Canada Pest Management
Regulatory Agency. December 22, 2006.
http://www.fluorideaction.org/pesticides/novaluron.canada.12-22-06.pdf
-- SUBCHRONIC STUDIES
(Oral) 52846-002; 174427; " 'Rimon' Technical: Toxicity Study
by Dietary Administration to CD Rats for 13 Weeks Followed by
a 4 Week Reversibility Period"; (P.W. East; Huntingdon Life
Sciences Ltd, Eye, Suffolk, England; Report No. MAK399/972319;
4/2/98); Ten CD rats/sex/group were fed 0, 50, 100, 10000 or 20000
ppm of Rimon Technical (Novaluron Technical) (batch no. 031068069,
purity: 99.8% (pre-study analysis)) in the diet for 13 weeks ((M):
0, 4.2, 8.3, 818.5, 1666.9 mg/kg/day, (F): 0, 4.7, 8.9, 871.0,
1820.6 mg/kg/day). An additional 5 animals/sex/group in the 0,
50 and 20000 ppm groups were maintained for 4 more weeks after
the termination of dosing in order to assess the reversibility
of treatment-related effects. No treatment-related mortality resulted.
No treatment-related effects on clinical signs, food consumption
or body weight were evident. The red blood
cell was the target of toxicity. The mean red blood cell
count was decreased in a dose-related manner
((M) 10000 ppm and above,
p<0.001 at 10000 ppm), (F)
50 ppm and above, p<0.05 at 50 ppm). Likewise, hemoglobin
content was decreased in a dose-related manner ((M)
10000 ppm and above, p<0.01 at 10000 ppm, (F)
100 ppm and above, p<0.001 at 100 ppm). For the females the packed
cell volume was lower for the 100 ppm treatment group and above
(p<0.001). In conjunction with these effects on the red blood
cells, the % of methemoglobin was increased
in the 10000 and 20000 ppm groups (p<0.001). As a response to
this effect, the % of reticulocytes was
increased in these two groups (p<0.05 or p<0.001)...
-- DERMAL: 52846-038; 178971; " 'Rimon' Technical: Toxicity
Study by Dermal Administration to CD Rats for 4 Weeks"; (P.B.
Rees; Huntingdon Life Sciences Ltd, Eye, Suffolk, England; Project
ID. MAK/478; 9/14/98); The skin of 5 CD rats/sex/group was treated
with 0, 75, 400 or 1000 mg/kg/day of RIMON Technical (batch no.
970211/4, purity: 99.7%) for 6 hours/day for 28 days. The test
material was suspended in 1.0% (w/v) aqueous methylcellulose.
No mortality resulted from the treatment. The mean body weight
and food consumption values for the 1000 mg/kg group males were
less than those of the control animals. The methemoglobin
concentration was greater for the 1000 mg/kg males (p<0.05)
and the 400 (p<0.01) and 1000 mg/kg (p<0.001) females. No treatment-related
effects were noted in the ophthalmology, clinical chemistry, or
urinalysis. There were no treatment-related lesions in either
the gross or microscopic examinations. No adverse effect indicated.
NOEL: (Systemic) (M) 400 mg/kg/day (based upon the lower mean
body weight and food consumption and increased
methemoglobin level noted for the 1000 mg/kg males) (F)
75 mg/kg/day (based upon increased methemoglobin
level noted for the 400 mg/kg females); (Dermal) 1000 mg/kg/day
(no effect evident at the highest dose tested). Study acceptable.
(Moore, 3/20/01)
Ref: 2001. Summary of Toxicology Data for
Novaluron. California Environmental Protection Agency, Department
of Pesticide Regulation, Medical Toxicology Branch. Chemical Code
# 5754, Tolerance # 52846 3/23/01.
http://www.fluoridealert.org/pesticides/Novaluron.CAepa.ToxTst.2001.pdf
--
Short-term incidental oral (1-30 days); Intermediate-term incidental
oral (1-6 months); and Intermediate-term
inhalation (1 to 6 months): 90-day feeding study in rat.
LOAEL = 8.64 mg/kg/day based on clinical chemistry (decreased
hemoglobin, hematocrit and RBC counts) and
histopathology (increased hematopoiesis and hemosiderosis in spleen
and liver).
Re:
June 2, 2004. Novaluron; Pesticide Tolerance. Final Rule. Federal
Register.
http://www.fluoridealert.org/pesticides/novaluron.fr.june.2.2004.htm
Body
Weight Decrease
(click on for all fluorinated
pesticides)
-- DERMAL: 52846-038;
178971; " 'Rimon' Technical: Toxicity Study by Dermal Administration
to CD Rats for 4 Weeks"; (P.B. Rees; Huntingdon Life Sciences
Ltd, Eye, Suffolk, England; Project ID. MAK/478; 9/14/98); The
skin of 5 CD rats/sex/group was treated with 0, 75, 400 or 1000
mg/kg/day of RIMON Technical (batch no. 970211/4, purity: 99.7%)
for 6 hours/day for 28 days. The test material was suspended in
1.0% (w/v) aqueous methylcellulose. No mortality resulted from
the treatment. The mean body weight
and food consumption values for the 1000 mg/kg group males
were less than those of the control animals. The
methemoglobin concentration was greater for the 1000 mg/kg males
(p<0.05) and the 400 (p<0.01) and 1000 mg/kg (p<0.001) females.
No treatment-related effects were noted in the ophthalmology,
clinical chemistry, or urinalysis. There were no treatment-related
lesions in either the gross or microscopic examinations. No adverse
effect indicated. NOEL: (Systemic) (M) 400 mg/kg/day (based
upon the lower mean body weight and food
consumption and increased methemoglobin level noted
for the 1000 mg/kg males) (F) 75 mg/kg/day (based upon increased
methemoglobin level noted for the 400 mg/kg females); (Dermal)
1000 mg/kg/day (no effect evident at the highest dose tested).
Study acceptable. (Moore, 3/20/01)
Ref: 2001. Summary of Toxicology Data for
Novaluron. California Environmental Protection Agency, Department
of Pesticide Regulation, Medical Toxicology Branch. Chemical Code
# 5754, Tolerance # 52846 3/23/01.
http://www.fluoridealert.org/pesticides/Novaluron.CAepa.ToxTst.2001.pdf
CNS
(click
on for all fluorinated pesticides)
52846-001; 174426;
" 'Rimon' Technical: Neurotoxicity Study by a Single Oral
Gavage Administration to CD Rats Followed by a 14-Day Observation
Period"; (A. Broadmeadow, W.D. Harvey and M.J. Collier; Huntingdon
Life Sciences Ltd, Huntingdon, Cambridgeshire, PE18 6ES, England;
Report No. MAK 480/983207; 2/3/99); Ten CD rats/sex/group were
dosed orally by gavage with 0, 200, 650 or 2000 mg/kg of Rimon
Technical (Novaluron Technical) (batch no. 970211/4; purity: 99.3%).
The animals were examined in the functional observational battery
(FOB) and motor activity assessments prior to dosing, on day 1
(at one hour post-dose) and on days 8 and 15. Five animals/sex/group
in the control and high dose group were chosen for histological
evaluation of the nervous system and muscle. No mortality resulted
from the treatment. The incidence of the clinical signs, piloerection
and irregular or fast breathing occurred in a dose-related manner
for all of the treatment groups between days 3 and 5 post-dose.
Among the parameters evaluated in the FOB and the motor activity
measurements, only the forelimb grip strength was apparently affected
by the treatment. The mean forelimb grip strength of the 2000
mg/kg males was less than that of the control animals at 1 hour
post-dose (p<0.05). There was an increased
incidence of degenerated fibers (minimal) in the peripheral nerves
of the high dose group (M: (0) 0/5 vs. (2000) 2/5, F: (0) 1/5
vs. (2000) 3/5). Possible adverse effect: increased incidence
of degenerated fibers in the peripheral nerves. The study
data were insufficient to establish a NOEL for neurotoxicity.
Acute NOEL: < 200 mg/kg (based upon the incidence of clinical
signs in the 200 mg/kg group). Study unacceptable, possibly upgradeable
to acceptable with the submission of histopathology data for the
200 and 650 mg/kg treatment groups. (Moore, 11/2/00)
Ref:
2001. Summary of Toxicology Data for Novaluron. California Environmental
Protection Agency, Department of Pesticide Regulation, Medical
Toxicology Branch. Chemical Code # 5754, Tolerance # 52846 3/23/01.
http://www.fluoridealert.org/pesticides/Novaluron.CAepa.ToxTst.2001.pdf
Liver
(click
on for all fluorinated pesticides)
--
Short-term incidental oral (1-30 days); Intermediate-term incidental
oral (1-6 months); and Intermediate-term
inhalation (1 to 6 months): 90-day feeding study in rat.
LOAEL = 8.64 mg/kg/day based on clinical chemistry (decreased
hemoglobin, hematocrit and RBC counts) and histopathology
(increased hematopoiesis and hemosiderosis in spleen and liver).
-- Chronic toxicity - dog.
NOAEL= 10 mg/kg/day. LOAEL=100 mg/kg/day
based on hematologic changes associated with
histopathological changes in liver
and spleen.
-- Short-term, Intermediate-term and Long-term
inhalation: LOAEL = 8.64 mg/kg/day based on clinical chemistry
(decreased hemoglobin, hematocrit and RBC counts) and histopathology
(increased hematopoiesis and hemosiderosis in spleen and liver)
Re:
June 2, 2004. Novaluron; Pesticide Tolerance. Final Rule. Federal
Register.
http://www.fluoridealert.org/pesticides/novaluron.fr.june.2.2004.htm
Reproductive
and Developmental (click
on for all fluorinated pesticides)
Excerpt from Table 1.--Subchronic, Chronic, and
Other Toxicity
-- Reproduction and
fertility- rat. NOAEL (M/F)= 74.2>=1009.8
mg/kg/day; LOAEL= 297.5 mg/kg/ day based
on decreased epididymal sperm counts and
increased age of preputial separation in the F1 generation,
reproductive LOAEL for females was not established.
Re:
June 2, 2004. Novaluron; Pesticide Tolerance. Final Rule. Federal
Register.
http://www.fluoridealert.org/pesticides/novaluron.fr.june.2.2004.htm
The NOAEL for developmental toxicity was also less than 74.2
mg/kg bw/day on the basis of decreased lactational
body weight gain, and increased spleen and liver weight in both
F1 and F2 pups observed at this dose level. When adult
and offspring spleen weights were compared, adults appeared to
be more sensitive to the effects of treatment. However, the offspring
would have received the treatment (via milk and a small portion
in diet during the latter portion of the lactation phase) for
21 days only, compared to the parental treatment duration of 17
weeks. The NOAEL for reproductive toxicity was set at 74.2 mg/kg
bw/day on the basis of decreased epididymal sperm count and delayed
sexual maturation in the F1 males at 297.5 mg/kg bw/day. Overall,
Novaluron was not considered to cause selective toxicity to the
developing young. (PAGE 13)
Ref: Proposed Registration Decision. PRD2006-05.
Health Canada Pest Management Regulatory Agency. December 22,
2006.
http://www.fluorideaction.org/pesticides/novaluron.canada.12-22-06.pdf
Spleen
(click
on for all fluorinated pesticides)
-- SUBCHRONIC STUDIES
(Oral) 52846-002; 174427; " 'Rimon' Technical: Toxicity Study
by Dietary Administration to CD Rats for 13 Weeks Followed by
a 4 Week Reversibility Period"; (P.W. East; Huntingdon Life
Sciences Ltd, Eye, Suffolk, England; Report No. MAK399/972319;
4/2/98); Ten CD rats/sex/group were fed 0, 50, 100, 10000 or 20000
ppm of Rimon Technical (Novaluron Technical) (batch no. 031068069,
purity: 99.8% (pre-study analysis)) in the diet for 13 weeks ((M):
0, 4.2, 8.3, 818.5, 1666.9 mg/kg/day, (F): 0, 4.7, 8.9, 871.0,
1820.6 mg/kg/day). An additional 5 animals/sex/group in the 0,
50 and 20000 ppm groups were maintained for 4 more weeks after
the termination of dosing in order to assess the reversibility
of treatment-related effects... Microscopic examination of the
spleen revealed increased extramedullary erythropoesis (50 ppm
and above) and increased hemosiderosis ((M)
10000 ppm and
above (p<0.01), (F)
50 ppm and above (p<0.05 at 50 ppm). In the livers
of the 10000 and 20000 ppm females, pigmented Kupffer cells were
noted (p<0.05 at 10000 ppm). At the conclusion of the 4 week recovery
period, the methemoglobin levels were still slightly elevated
for the 20000 ppm group (p<0.05), the relative spleen
weight was increased for the 20000 ppm females (p<0.05),
and there was still increased hemosiderosis
in the spleen of the 20000 ppm females (p<0.01). No adverse
effect indicated. NOEL: (M/F) < 50 ppm ((M) 4.2 mg/kg/day, (F)
4.7 mg/kg/day (based upon the increased incidence of splenic
extramedullary erythropoesis noted for the 50 ppm treatment
group); Study acceptable. (Moore, 11/1/00)
Ref: 2001. Summary of Toxicology Data for
Novaluron. California Environmental Protection Agency, Department
of Pesticide Regulation, Medical Toxicology Branch. Chemical Code
# 5754, Tolerance # 52846 3/23/01.
http://www.fluoridealert.org/pesticides/Novaluron.CAepa.ToxTst.2001.pdf
--
Short-term incidental oral (1-30 days);
Intermediate-term incidental oral (1-6 months); and Intermediate-term
inhalation (1 to 6 months): 90-day feeding study in rat.
LOAEL = 8.64 mg/kg/day based on clinical chemistry (decreased
hemoglobin, hematocrit and RBC counts) and histopathology
(increased hematopoiesis and hemosiderosis in spleen and liver).
-- Reproduction and fertility-
rat. Parental NOAEL= Not established;
LOAEL
(M/F)= 74.2/84.0 mg/kg/day based on increased
absolute and relative spleen weights. Offspring
NOAEL= Not established; LOAEL (M/F)= 74.2/84.0 mg/
kg/day based on increased absolute
and relative spleen weights.
Re:
June 2, 2004. Novaluron; Pesticide Tolerance. Final Rule. Federal
Register.
http://www.fluoridealert.org/pesticides/novaluron.fr.june.2.2004.htm
Testes
(click
on for all fluorinated pesticides)
Excerpt from Table 1.--Subchronic, Chronic, and
Other Toxicity
-- Reproduction and
fertility- rat. NOAEL (M/F)= 74.2>=1009.8
mg/kg/day; LOAEL= 297.5 mg/kg/ day based
on decreased epididymal sperm counts
and increased age of preputial separation in the F1 generation,
reproductive LOAEL for females was not established.
Re:
June 2, 2004. Novaluron; Pesticide Tolerance. Final Rule. Federal
Register.
http://www.fluoridealert.org/pesticides/novaluron.fr.june.2.2004.htm
| Environmental
(click
on for all fluorinated pesticides)
PAGE 30: ...Novaluron
is bioaccumulative. The n-octanol–water partition
coefficient (log Kow) is 4.3, which is below the TSMP Track-1
cut-off criterion of $5.0. However, studies have determined
that the bioconcentration factor (BCF) is between 14220x
and 14645x that of the concentration in water, which is
greater than the TSMP Track-1 cut-off criterion of BCF ≥
5000.
PAGE 4: Novaluron
presents high risks to freshwater and marine aquatic invertebrates,
and moderate risk to marine mollusks. There is also
some risk to susceptible non-target plant species. Beneficial
insects, such as, predatory mites, parasitoid wasps, and
honeybees may be temporarily suppressed. Therefore, hazard
statements and specific instructions to reduce spray drift
to terrestrial insects are provided on the product label.
Depending on the type of application equipment, timing of
spray, and crop, the buffer zones may vary from 3 to 80
metres for freshwater/estuarine aquatic organisms, and 1
to 30 metres for non-target terrestrial plant species.
PAGE 26: The aquatic risk assessment of
novaluron, Rimon 10 EC, and 275-352I identified areas of
concern, particularly with aquatic invertebrates (benthic
and pelagic) and marine mollusks. Therefore, mitigation
measures, by way of buffer zones and environmental hazard
statements, are required for the protection of these organisms.
The most sensitive endpoint chosen for the buffer zone calculations
was the chronic daphnia. This endpoint was chosen because
it affords greater protection for freshwater benthic invertebrates
(gammarids), which were more sensitive than daphnia in a
community microcosm study. In this study, the daphnia fully
recovered, however, gammarids were completely eliminated
and experienced no recovery whatsoever. Buffer zones to
protect aquatic organisms range from 3 to 80 metres, depending
on the crop, application method, type of habitat, and stage
of growth.
PAGE 20: ... A log
Kow of 4.3 indicates the potential for novaluron bioaccumulation,
which is supported by two bioconcentration studies. In these
studies, novaluron was readily accumulated by fish during
exposure. Novaluron steady state concentrations were attained
within 21-35 days, with bioconcentration factors of 14220-14645x
for the whole body. The clearance pattern from the whole
body was first-order with half-lives of 11-14 days. Approximately
40 days were required for 95% novaluron depuration from
the whole body. The relatively high level of novaluron bioconcentration
by fish, its resistance to significant transformation and
its slow rate of loss during depuration suggest that it
may have some potential for persistence in the aquatic food
chain, particularly when frequent applications are made.
PAGES 25-26:... Using the corresponding
expected concentration of novaluron in water still led to
risk quotients higher than one for all organisms identified
as being at risk under a worst-case scenario (Table 4.4,
Appendix I). Further refinement in the assessment involved
the determination of risk at the novaluron solubility limit
(3.4 ?g/L), as free-swimming aquatic organisms are not expected
to be exposed to concentrations beyond this value. Even
at novaluron concentrations as low as the solubility limit
(3.4 ?g/L), however, aquatic invertebrates, mollusks, and
microcosm communities continue to be at risk from acute
and/or chronic exposures with risk quotients greater than
one by one to two orders of magnitude (Table 4.4, Appendix
I). Following the risks of novaluron toxicity to aquatic
invertebrates and mollusks at the solubility limit, a refined
assessment was conducted. Accordingly, the most likely routes
of novaluron entry into water are primarily through drift
and surface water runoff. Therefore, risks to these above
organisms were determined at expected environmental concentrations
based on a runoff simulation model, with 10% drift taken
into account, for apple/pear and potato applications. The
simulated water body consisted of a 1 ha wetland with an
average depth of 80 cm and a 10 ha drainage area. From the
simulation, peak concentrations for apple/pear and potato
applications in runoff water were 1.64 and 0.77 ?g a.i./L,
respectively 6. Corresponding peak sediment interstitial
water concentrations were 113 and 77 ng a.i./L 7. At these
expected concentrations, free-swimming aquatic invertebrates,
such as daphnia, remain at risk to novaluron, resulting
from acute and chronic exposures, at both apple/pear and
potato application rates; risk quotients range from 3.8
to 8.4 (Table 4.4, Appendix I). Based on both free-water
and sediment interstitial pore-water concentration simulations,
risks also remain for significant impacts to microcosm communities,
including both free-swimming and sediment-dwelling aquatic
invertebrates (pelagic and benthic), resulting immediately
after a simulated application (peak) and after longer term
exposures (>90 days) for apple/pear and potato uses;
risk quotients range from 1.3 to 33 (Table 4.4, Appendix
I). There is risk to marine mollusks, after 21 days of exposure
simulation, only when novaluron is applied on potatoes.
In general, aquatic invertebrates that live in or on freshwater
lake, river, or estuarine sediments are particularly susceptible
and at risk considering that all aspects of their life cycles
are spent in direct contact with sediment and associated
interstitial water for an indefinite period of time, and
that novaluron rapidly dissipates from the water phase to
the sediment phase (Figure 4.1, Appendix I).
Ref: Proposed Registration
Decision. PRD2006-05. Health Canada Pest Management Regulatory
Agency. December 22, 2006.
http://www.fluorideaction.org/pesticides/novaluron.canada.12-22-06.pdf
|
|

