|
Return
to LPOS Index Page
Activity:
Insecticide, Adjuvant
(unclassified)
Structure:
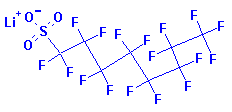
Adverse
Effects:
Blood
Body
Weight Decrease
Bone
(including
Cleft Palate)
Embryotoxic
Fetotoxic
Liver
Environmental
See PFOA
perfluorinated chemicals
Lithium
perfluorooctane sulfonate is classified as a PFOS. It is
one of several hundred PFOS chemicals that were produced
mainly by 3M. [One of the most recognized uses was as a
stain repellant in SCOTCHGUARD.] In 2000, 3M announced that
it would end the production of approximately 150 PFOS chemicals.
At this same time US EPA was committed to a 100% phase out
of all PFOS. However, a few industries have successfully
petitioned for the use of small amounts due to lack of alternaitves.
We assume that sometime since 2001 Lithium perfluorooctane
sulfonate use as a pesticide was ended. However, this is
just an assumption, as manufacturers may have been allowed
to use up their stock, instead of destroying it (it is unknown
as to whether PFOS can be destroyed. There is information
that suggests it will be reduced to its most toxic congener).
"The
PFOS story is likely to emerge as one of the apocryphal
examples of 20th century experimentation
with
widespread chemical exposures:
prolific use and almost no testing for safety, until unexpectedly
and almost serendipitously, it is discovered as a contaminant
virtually everywhere. And
as is often the case in these stories, the company producing
PFOS products possessed information hinting at its risks
but chose not to share their data with regulators or the
public for years...
Ref: Our Stolen Future website
- http://www.ourstolenfuture.org/NewScience/oncompounds/PFOS/2001-04pfosproblems.htm
|
Note from FAN:
The following data is specific to Lithium perfluorooctane sulfonate
(LPOS). In February we will have a PFOS section with a link available
at this site.
Blood
(click
on for all fluorinated pesticides)
-- Based on the findings
of the 90-day subchronic toxicity study, it appears that LPOS
suppresses the formation of blood in males.
This endpoint is not of concern because subchronic or chronic
exposure is not expected from this use. Based on the results of
the developmental studies, there does not appear to be any increased
sensitivity of the pups in comparison to the maternal parents.
-- In an unacceptable subchronic oral toxicity study in rats,
the LOAEL was found to be 0.60 mg/kg/day in females, based on
increased liver weight and 0.30 mg/kg/day in males, based on decreased
triglycerides and hepatocytic vacuolization. The NOAEL is 0.20
mg/kg/day in females. No NOAEL was determined in males.
It appears that LPOS suppresses hematopoiesis
(blood production) in males as indicated by significant decreases
in RBCs, hemoglobin, hematocrit, and the finding of extramedullary
hematopoiesis. This study was classified as unacceptable
for the following reasons: 1) a NOAEL was not determined in males;
2) an analysis of blood coagulation factors which is required
for a subchronic study was not measured in the animals; 3) the
mean food and water consumption calculated as g/week is not correct
in the study (it is actually g/day) which raises the possibilities
of potential errors in compound consumption, since the test substance
was administered in drinking water; and 4) a range finding study
was not performed in order to develop a rationale for the appropriate
selection of doses in males and females.
Ref: US EPA. New Pesticide Fact Sheet. Lithium
perfluorooctane sulfonate (LPOs). August 1999.
http://www.epa.gov/opprd001/factsheets/lithium.pdf
Body
Weight Decrease
(click
on for all fluorinated pesticides)
Abstract: Lithium Perfluorooctane
sulfonate (LPOS) was administered by gavage at 3, 6, or 12 mg/kg
to mated Crl:CD„BR VAF/Plus„ female rats once daily on days 6
through 15 of gestation. Body weights and clinical observations
were on days 0, 6, 9, 12, 16, 20 of gestation. Food consumption
was also measured. Cesarean sections were done on surviving animals
on day 20 of gestation, and the fetuses were removed for examination.
The dams were necropsied following sacrifice. Clear maternal toxicity
was observed in both the 6 and 12 mg/kg groups. Five out of 25
females in the 12 mg/kg group did not survive to scheduled sacrifice.
Both the 6 and 12 mg/kg groups had test material-related changes
including lower mean body weights,
body weight gains, and food consumption. Treatment at 12 mg/kg
resulted in embryolethality as evidenced by lower uterine weights,
fewer live fetuses per litter, reduced fetal
bodyweights and lower percent of live fetuses than the
control treated. There was also significant increased incidences
of cleft palate (79%), and edema (36%). Variations at this dose
included reduced ossification of bone and unossified bone. The
no-observable-effect level (NOEL) for LPOS for teratogenicity
in rats is 6 mg/kg, whereas the NOEL for maternal toxicity in
rats is 3 mg/kg.
Ref: Henwood SM et al. (1994). Developmental
toxicity study with lithium perfluorooctane sulfonate in rats.
Toxicologist 1994 Mar;14(1):162.
-- Developmental Toxicity
In the developmental study in rabbits, maternal toxicity was observed
at 1 mg/kg/day and above, based on
reduced body weight gains during
the dosing period, followed by a rebound in body weight gains
post-dosing. Developmental toxicity was observed at the highest
dose tested, 4 mg/kg/day. Effects included fetolethality, skeletal
variations (unossified skull bones, sternebrae, talus, pubis and
extra full rib) and decreased fetal
body weights. A maternal NOAEL was not established and
the maternal LOAEL was 1 mg/kg/day, based on
reduced body weight gains. The developmental NOAEL was
2 mg/kg/day.
-- In the rat developmental study, maternal toxicity was observed
at 6 mg/kg/day based on reductions in mean
body weights, mean body weight gains, food
consumption and clinical signs (hunched and few
feces in one animal). The maternal NOAEL is 3 mg/kg/day. The developmental
NOAEL is 6 mg/kg/day and the LOAEL is 12 mg/kg/day based on increased
fetolethality, lower fetal body weights,
external and soft tissue malformations, and skeletal
variations.
Ref: US EPA. New Pesticide Fact Sheet. Lithium
perfluorooctane sulfonate (LPOs). August 1999.
http://www.epa.gov/opprd001/factsheets/lithium.pdf
Bone
(click
on for all fluorinated pesticides)
Developmental Toxicity.
In the developmental study in rabbits, maternal toxicity was observed
at 1 mg/kg/day and above, based on reduced body weight gains during
the dosing period, followed by a rebound in body weight gains
post-dosing. Developmental toxicity was observed at the highest
dose tested, 4 mg/kg/day. Effects included fetolethality, skeletal
variations (unossified skull bones,
sternebrae, talus, pubis and extra full rib) and decreased
fetal body weights. A maternal NOAEL was not established and the
maternal LOAEL was 1 mg/kg/day, based on reduced body weight gains.
The developmental NOAEL was 2 mg/kg/day... In the rat developmental
study, maternal toxicity was observed at 6 mg/kg/day based on
reductions in mean body weights, mean body weight gains, food
consumption and clinical signs (hunched and few feces in one animal).
The maternal NOAEL is 3 mg/kg/day. The developmental NOAEL is
6 mg/kg/day and the LOAEL is 12 mg/kg/day based on increased fetolethality,
lower fetal body weights, external and soft tissue malformations,
and skeletal variations.
Ref:
US EPA. New Pesticide Factsheet. Lithium
Perfluorooctane sulfonate (LPOS). August 1990. EPA-730-F-99-009.
http://www.fluoridealert.org/pesticides/Lithium.per..EPA.FACTS.1999.pdf
Abstract (rat):
Lithium Perfluorooctane sulfonate (LPOS) was administered by gavage
at 3, 6, or 12 mg/kg to mated Crl:CD„BR VAF/Plus„ female rats
once daily on days 6 through 15 of gestation. Body weights and
clinical observations were on days 0, 6, 9, 12, 16, 20 of gestation.
Food consumption was also measured. Cesarean sections were done
on surviving animals on day 20 of gestation, and the fetuses were
removed for examination. The dams were necropsied following sacrifice.
Clear maternal toxicity was observed in both the 6 and 12 mg/kg
groups. Five out of 25 females in the 12 mg/kg group did not survive
to scheduled sacrifice. Both the 6 and 12 mg/kg groups had test
material-related changes including lower mean body weights, body
weight gains, and food consumption. Treatment
at 12 mg/kg resulted in embryolethality as evidenced by lower
uterine weights, fewer live fetuses per litter, reduced fetal
bodyweights and lower percent of live fetuses than the control
treated. There was also significant increased incidences
of cleft palate (79%), and edema (36%).
Variations at this dose included reduced ossification of bone
and unossified bone. The no-observable-effect level (NOEL)
for LPOS for teratogenicity in rats is 6 mg/kg, whereas the NOEL
for maternal toxicity in rats is 3 mg/kg.
Ref: Toxicologist
1994 Mar;14(1):162; Developmental
toxicity study with lithium perfluorooctane sulfonate in
rats; by Henwood
SM, Costello AC, Osimitz TG
Abstract (rabbit):
Lithium perfluorooctane sulfonate (LPOS) was administered by gavage
at 1, 2, or 4 mg/kg to mated New Zealand White female rabbits
once daily on Days 7 through 19 of gestation. Body weights and
clinical observations were made on Days 0, 7, 10, 13, 16, 20,
24, and 29 of gestation. Cesarean sections were done on surviving
animals on Day 29 of gestation, and the fetuses were removed for
examination. The does were necropsied following sacrifice. Treatment
at the 4-mg/kg level resulted in abortions; premature deliveries;
and lower mean body weights, lower body weight gains, and lower
gravid uterine weights than those of the control groups. Cesarean
sections revealed a treatment-related increase in postimplantation
losses at the 4-mg/kg level. Mean fetal body weight (mean = 10.01
g, n = 11) at the 4-mg/kg level was lower than that of controls
(mean = 39.93 g, n = 16) and represents developmental toxicity.
Fetal morphological examinations disclosed no evidence of teratogenicity
of LPOS in rabbits at any level. There were increased incidences
of unossified skeletal structures at the
2- and 4-mg/kg levels. These variations along with the lower fetal
body weights suggest retarded development at the 4-mg/kg level.
The no-observable-effect level of LPOS for developmental toxicity
in rabbits is considered to be 2 mg/kg.
Ref: Henwood SM et al. (1994). Developmental
toxicity study with lithium perfluorooctane sulfonate in
rabbits.
Teratology 1994 May;49(5):398.
Embryotoxic
(click
on for all fluorinated pesticides)
Abstract (rat):
Lithium Perfluorooctane sulfonate (LPOS) was administered by gavage
at 3, 6, or 12 mg/kg to mated Crl:CD„BR VAF/Plus„ female rats
once daily on days 6 through 15 of gestation. Body weights and
clinical observations were on days 0, 6, 9, 12, 16, 20 of gestation.
Food consumption was also measured. Cesarean sections were done
on surviving animals on day 20 of gestation, and the fetuses were
removed for examination. The dams were necropsied following sacrifice.
Clear maternal toxicity was observed in both the 6 and 12 mg/kg
groups. Five out of 25 females in the 12 mg/kg group did not survive
to scheduled sacrifice. Both the 6 and 12 mg/kg groups had test
material-related changes including lower mean body weights, body
weight gains, and food consumption. Treatment
at 12 mg/kg resulted in embryolethality as evidenced by lower
uterine weights, fewer live fetuses per litter, reduced fetal
bodyweights and lower percent of live fetuses than the control
treated. There was also significant
increased incidences of cleft palate (79%), and edema (36%). Variations
at this dose included reduced ossification of bone and unossified
bone. The no-observable-effect level (NOEL) for LPOS for
teratogenicity in rats is 6 mg/kg, whereas the NOEL for maternal
toxicity in rats is 3 mg/kg.
Ref: Toxicologist
1994 Mar;14(1):162; Developmental
toxicity study with lithium perfluorooctane sulfonate in
rats; by Henwood
SM, Costello AC, Osimitz TG
Fetotoxic
(click on for all fluorinated pesticides)
Developmental Toxicity
In the developmental study in rabbits, maternal toxicity was observed
at 1 mg/kg/day and above, based on reduced body weight gains during
the dosing period, followed by a rebound in body weight gains
post-dosing. Developmental toxicity was observed at the highest
dose tested, 4 mg/kg/day. Effects included fetolethality,
skeletal variations (unossified skull bones, sternebrae, talus,
pubis and extra full rib) and decreased fetal body weights. A
maternal NOAEL was not established and the maternal LOAEL was
1 mg/kg/day, based on reduced body weight gains. The developmental
NOAEL was 2 mg/kg/day. In the rat developmental study, maternal
toxicity was observed at 6 mg/kg/day based on reductions in mean
body weights, mean body weight gains, food consumption and clinical
signs (hunched and few feces in one animal). The maternal NOAEL
is 3 mg/kg/day. The developmental NOAEL is 6 mg/kg/day and the
LOAEL is 12 mg/kg/day based on increased
fetolethality, lower fetal body weights, external and soft tissue
malformations, and skeletal variations.
Ref:
US EPA. New Pesticide Factsheet. Lithium
Perfluorooctane sulfonate (LPOS). August 1990. EPA-730-F-99-009.
http://www.fluoridealert.org/pesticides/Lithium.per..EPA.FACTS.1999.pdf
Liver
(click on for all fluorinated pesticides)
In an unacceptable
subchronic oral toxicity study in rats, the LOAEL
was found to be 0.60 mg/kg/day in females, based on increased
liver weight and 0.30 mg/kg/day in males, based on
decreased
triglycerides and hepatocytic vacuolization.
The NOAEL is 0.20 mg/kg/day in females.
No NOAEL was determined in males. It appears that
LPOS suppresses hematopoiesis (blood production) in males as indicated
by significant decreases in RBCs, hemoglobin, hematocrit, and
the finding of extramedullary hematopoiesis. This study
was classified as unacceptable for the following reasons: 1) a
NOAEL was not determined in males; 2) an analysis of blood coagulation
factors which is required for a subchronic study was not measured
in the animals; 3) the mean food and water consumption calculated
as g/week is not correct in the study (it is actually g/day) which
raises the possibilities of potential errors in compound consumption,
since the test substance was administered in drinking water; and
4) a range finding study was not performed in order to develop
a rationale for the appropriate selection of doses in males and
females.
Ref: US EPA. New Pesticide Fact Sheet. Lithium
perfluorooctane sulfonate (LPOs). August 1999.
http://www.epa.gov/opprd001/factsheets/lithium.pdf
| Environmental
(click
on for all fluorinated pesticides)
--
The proposed use pattern for LPOS poses minimal to no aquatic
or terrestrial animal exposure. Therefore, a risk assessment
was not performed.. The end-use bait product may be attractive
to honey bees. However, honeybee exposure is expected to
be minimal because the bait unit contains a pad which soaks
up the chemical material and makes it available to wasps
and hornets, but not bees. (Wasps have sponge-like mouthparts
for lapping up liquid food, but bees have an elongated tonguelike
structure for sucking up liquid food). The
proposed label cautions that the product is highly toxic
to honey bees, and baits should be placed in areas
away from flowers to mitigate attraction and exposure. EPA
found the labeling precaution to be adequate.
-- Acceptable acute oral toxicity studies were submitted
to establish the toxicity of LPOS to birds. Results are
tabulated below. Species LD50 Toxicity Category Northern
bobwhite quail (Colinus virginianus) 42 (mg/kg) - highly
toxic. Mallard duck (Anas platyrhynchos) 81(mg/kg) moderately
toxic. Since one of the LD50 values falls in the range of
10 - 50 mg/kg, LPOS is categorized
as highly toxic to avian species on an acute oral basis.
-- Two acceptable subacute dietary
studies were submitted to establish the toxicity of LPOS
to birds. The preferred test species are mallard duck and
bobwhite quail. Results of these tests are tabulated below.
Species 5-Day LC50 (ppm) Toxicity Category Northern bobwhite
quail (Colinus virginianus) 220
(ppm) - highly toxic.
Mallard duck (Anas platyrhynchos) 324
(ppm) - highly toxic.
Since the LC50 falls in the range of 51 - 500 ppm, lithium
perfluorooctane sulfonate is categorized as highly
toxic to avian species on a subacute dietary basis.
-- Insects Since its intended use
is to control related hymenopterons (wasps, hornets and
yellow jackets) there is a potential for honey bee exposure.
A honey bee acute contact study is not available for LPOS.
However, results of efficacy testing on a hornet, Dolichovespula
maculata, show a that a mean concentration of 1.5 ug per
bee resulted in 93% mortality within 29 hours. These results
indicate that LPOS is categorized as very highly
toxic to bees on an acute contact basis. A
honey bee acute contact study is not required, since the
results of the efficacy test have been deemed adequate to
demonstrate toxicity to honey bees.
Ref: US EPA. New Pesticide
Fact Sheet. Lithium perfluorooctane sulfonate (LPOs). August
1999. http://www.epa.gov/opprd001/factsheets/lithium.pdf
The
substance shows moderate acute toxicity to aquatic organisms,
the lowest LC50 for fish is a 96-hour LC50 of 4.7 mg/l to
the fathead minnow Pimephales promelas for the lithium salt.
Ref:
November 21, 2002 report:
Hazard Assessment of Perfluorooctane sulfonate (PFOS) and
its salts.
Organisation for Economic Co-operation and Development.
ENV/JM/RD(2002)17/FINAL.
http://www.fluorideaction.org/pesticides/pfos.final.report.nov.2002.pdf
|
|

