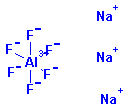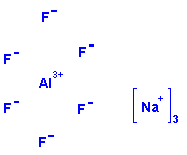|
Return to Cryolite
Index Page
See
Cryolite Abstracts
ACTIVITY: Insecticide
(Fluorine, Inorganic)
Structure:
Adverse Effects:
Anemia
Body Weight Decrease - Anorexia, Wasting
Bone
Stomach
Click
here to see:
Table
1. Top 50 Crops and Sites for for Cryolite use in California
in 2002.
Table 2. Use by county in California for Cryolite on
All Sites in 2002.
Table A. PAN's Explanation of Terms
Table
3. Cryolite
Pesticide Use in California:
1991-2000
Table
4. 1992
- Estimated Cryolite Use in US
(including map)
|
| As
of February 13, 2005, this insecticide is permitted in
or on 32 food commodities in the United States -
see list at http://www.fluorideaction.org/pesticides/mrl.cryolite.htm
--
its main use is in the production of aluminum where it forms
the electrolytic bath. It has also found industrial use in
the production of insecticides, metals and alloys, glass and
enamels, welding rods, resins, explosives and fireworks, and
polishes. The toxic effects of cryolite are largely due to
its content of aluminum and fluoride. Thus,
its toxic effects, if not known, have
to be based on known adverse effects of aluminum and fluoride.
Ref: Authors: Soderlund E (1995). Health effects of selected
chemicals 3. Cryolite (sodium aluminium fluoride). Source:
TA:Nord PG:28-50 YR:1995 IP: VI:28
Note
from FAN:
For
a review of the risks associated with exposure to Cryolite
see: Comments
submitted to EPA on Gowan Company's petition for new, modified,
and proposed tolerances (Federal Register, April 24, 2002),
submitted by Paul and Ellen Connett on May 24, 2002. Some
excerpts:
When the EPA treats cryolite simply as a
source of fluoride they oversimplify the chemistry. In addition
to fluoride there will be free aluminum ions present or
intermediate aluminum-fluoride complexes. There are several
lines of scientific evidence to suggest that fluoride in
the presence of aluminum is a far greater concern than fluoride
alone. In this respect one key experiment was conducted
by Varner et al. (1998) which showed greater impacts on
the brain with rats treated with aluminum fluoride at 1
ppm fluoride than sodium fluoride at 1 ppm fluoride. This
study is discussed elsewhere in this critique.
There are several experiments reported in the literature
where fluoride in the presence of a trace amount of aluminum
triggers the G-protein messenger transmission system for
water soluble hormones, neural transmitters,
and certain growth factors. This is potentially extremely
serious and is discussed elsewhere in this critique.
Gowan's
petition states
...
the Agency has determined that although, fluoride accumulation
is demonstrated in a number of studies, the accumulation
itself is not considered an adverse effect.
Anything
that accumulates in the human body is potentially dangerous.
This is why the body has mechanisms to get rid of fat soluble
toxins otherwise they accumulate in fat. And that is why
water soluble substances like fluoride are excreted through
the kidney. However, it's generally accepted that 50% of
the fluoride (for healthy people under fifty years of age
-ATSDR, 1993, p 112) is not excreted and accumulates in
the bone. It would be reckless to assume that fluoride accumulation
from ALL sources that we are exposed to including pesticide
residues will not cause deleterious effects on the bone
and the pineal gland.
Until
Jennifer Luke's work (1997, 2001) many people were unaware
that the pineal gland produced the same crystals of calcium
hydroxy apatite as the bones and teeth. It is shocking that
no U.S. agency has yet to address Luke's studies in any
public statement or peer reviewed document.
Luke's
work is particularly illuminating in this respect because
not only did she show that fluoride accumulated in the human
pineal gland but she also showed that it lowered the production
of melatonin in animal studies, the hormone that is produced
in this gland.
Luke
also noted a finding from the first 10-year follow-up health
study of the Newburgh-Kingston fluoridation trial (which
was not thought significant at the time) that on average
the girls in Newburgh started menstruating 5 months earlier
than the girls in the control, non-fluoridated, city of
Kingston (Schlesinger et al., 1956). Thus one of the risks
we may be taking by exposing our whole population to fluoride
is interfering with delicate regulatory timing processes,
from the onset of puberty to the aging process...
Varner
JA et al. (1998). Chronic administration of aluminum-fluoride
or sodium-fluoride to rats in drinking water: alterations
in neuronal and cerebrovascular integrity. Brain Research,
784, 284-298.
ATSDR
(1993). Toxicological profile for fluorides, hydrogen fluoride,
and fluorine (F). U.S. Department of Health and Human Services,
Public Health Service, Agency for Toxic Substances and Disease
Registry. TP-91/17. Available online at:
http://www.fluoridealert.org/ATSDR-fluoride.pdf
Luke,
J (1997). The Effect of Fluoride on the Physiology of the
Pineal Gland. Ph.D. Thesis. University of Surrey, Guildord,
U.K.
Luke
J (2001). Fluoride deposition in the aged human pineal gland.
Caries Res. 35:125-128.
Schlesinger
ER et al . (1956). Newburgh-Kingston caries-fluorine study
X111. Pediatric findings after ten
years. Journal of the American Dental Association.
V 52.
|
Anemia
(click on for all fluorinated
pesticides)
-- CHRONIC FLUORINE
POISONING OCCURS AMONG MINERS OF CRYOLITE/ LOSS OF WEIGHT, ANOREXIA,
ANEMIA, WASTING ... AND DENTAL DEFECTS
ARE AMONG COMMON FINDINGS IN CHRONIC FLUORINE POISONING. THERE
MAY BE AN EOSINOPHILIA, AND IMPAIRMENT OF GROWTH IN YOUNG WORKERS.
SYMPTOMS OF INTOXICATION INCLUDE GASTRIC, INTESTINAL, CIRCULATORY,
RESP AND NERVOUS COMPLAINTS AND SKIN RASHES. [Sax, N.I. Dangerous
Properties of Industrial Materials. 6th ed. New York, NY: Van
Nostrand Reinhold, 1984. 1427]
-- Chronic poisoning: Intake of more than 6 mg of fluoride per
day results in fluorosis. Symptoms are weight loss, brittleness
of bones, anemia, weakness, general
ill health, stiffness of joints. ... /Fluoride/ [Dreisbach, R.
H. Handbook of Poisoning. 9th ed. Los Altos, California: Lange
Medical Publications, 1977. 207]
Ref: TOXNET from Hazardous Substances Data
Bank for ALUMINUM SODIUM FLUORIDE (Cryolite).
http://www.fluoridealert.org/pesticides/cryolite.toxnet.hsdb.htm
Body
Weight Decrease
(click
on for all fluorinated pesticides)
-- CHRONIC FLUORINE
POISONING OCCURS AMONG MINERS OF CRYOLITE/ LOSS
OF WEIGHT, ANOREXIA, ANEMIA,
WASTING ... AND DENTAL DEFECTS ARE
AMONG COMMON FINDINGS IN CHRONIC FLUORINE POISONING. THERE MAY
BE AN EOSINOPHILIA, AND IMPAIRMENT OF GROWTH
IN YOUNG WORKERS. SYMPTOMS OF INTOXICATION INCLUDE GASTRIC,
INTESTINAL, CIRCULATORY, RESP AND NERVOUS COMPLAINTS AND SKIN
RASHES. [Sax, N.I. Dangerous Properties of Industrial Materials.
6th ed. New York, NY: Van Nostrand Reinhold, 1984. 1427]
-- Chronic poisoning: Intake of more than 6 mg of fluoride per
day results in fluorosis. Symptoms are weight
loss, brittleness of bones, anemia, weakness, general ill
health, stiffness of joints. ... /Fluoride/ [Dreisbach, R. H.
Handbook of Poisoning. 9th
ed. Los Altos, California: Lange Medical Publications, 1977. 207]
Ref: TOXNET from Hazardous Substances Data
Bank for ALUMINUM SODIUM FLUORIDE (Cryolite).
http://www.fluoridealert.org/pesticides/cryolite.toxnet.hsdb.htm
Bone
(click
on for all fluorinated pesticides)
-- Exposure to cryolite
dust may result in skeletal fluorosis.
Eight male workers at a cryolite concentrator participated in
a 4 day study after 5 days of vacation. Dust exposures were 0.16
to 21.2 mg/cu m. Urine was collected before work began and during
two 4 hr periods. Preshift urine fluoride concentrations increased
during the week. Fluoride concentrations in postshift urine and
serum both correlated with the dust exposures. Serum fluoride
concentrations decreased with a half-life of 3.3 to 6.9 hr after
work. Fluoride clearance was 40.5 ml/min at urinary flow rates
of 0.89 to 2.21 ml/min. Serum aluminum concentrations varied without
relation to the exposure, but the urinary aluminum excretion correlated
with the fluoride levels. Preshift serum phosphate concentrations
increased significantly during the week, possibly indicating changes
in mineral metabolism. For monitoring of individual uptake of
cryolite dust, serum fluoride measurements are most useful. [Grandjean
P et al; J Occup Med 32 (10: 58-63 (1990)]
-- FROM ANALYSIS OF BONES OF 2 /CRYOLITE/
WORKERS ... /IT WAS/ ESTIMATED THAT THEIR SKELETAL
SYSTEMS CONTAINED 50 AND 90 G OF FLUORINE, RESPECTIVELY. THE LATTER
AMT HAD BEEN DEPOSITED DURING 7500 WORKING DAYS, CORRESPONDING
TO AN AVG DEPOSITION OF 12 MG/DAY. [Patty,
F. (ed.). Industrial Hygiene and Toxicology: Volume II: Toxicology.
2nd ed. New York: Interscience Publishers, 1963. 841]
-- Fluoride levels in urine should be checked periodically and
all workers should be subjected to periodical skeletal
X-ray exam particularly of the pelvis. /Fluoride and cmpd/
[International Labour Office. Encyclopedia of Occupational Health
and Safety. Vols. I&II. Geneva, Switzerland: International Labour
Office, 1983. 894]
Ref: TOXNET from Hazardous Substances Data
Bank for ALUMINUM SODIUM FLUORIDE, CAS No. 15096-52-3
http://www.fluoridealert.org/pesticides/cryolite.toxnet.hsdb.htm
6. A developmental
toxicity study conducted in female mice with cryolite at dose
levels of 0, 30, 100 and 300 mg/kg/day (gavage). The NOEL for
maternal toxicity is 30 mg/kg/day and the LOEL is 100 mg/kg/day
based on the occurrence of dark red contents of the stomach. Fetuses
at 300 mg/kg/day exhibited bent ribs and
bent limb bones.
The NOEL for developmental toxicity is 100 mg/kg/day. The LOEL
is 300 mg/kg/day based on an increase in
bent ribs and bent limbs.
Ref: Federal Register: May 8, 1996. Fluorine
Compounds; Pesticide Tolerance and Feed Additive Regulation. Proposed
Rule.
http://www.fluoridealert.org/pesticides/cryolite.fr.may.8.1996.htm
-- 145-045 139642 Nemec,
M.D., "Developmental toxicity study of Kryocide* in mice", WIL
Research Laboratories, Inc. Study No. WIL-160004, Jan. 6, 1992.
Kryocide*, purity of 97.3%, was administered via gavage at concentrations
of 0 (0.5% Methylcellulose), 100, 300 or 1000 mg/kg/day to 30
mated Crl:CD-1* (ICR) BR mice/group during gestation days 6 through
15. Maternal toxicity NOEL = 100 mg/kg/day. Mortality was 40%
and 10% for high and mid dose groups, respectively, with occasional
necropsy reporting of "red stomach contents"
or "reddened adrenals". Food consumption and body weight gains
were reduced at 1000 mg/kg/day. Survival was too low at 1000 mg/kg/day
to meaningfully assess treatment effects on fetuses, however a
small increase in incidences of cleft palate
and a single incident of open eyelid contributed toward a general
increase in malformations in this group. There were no definitive
developmental effects at or below 300 mg/kg/day, however a single
incident of the variation "bent ribs"
was considered an equivocal indication of a treatment effect,
so that 100 mg/kg/day is the developmental NOEL. This study is
not independently acceptable, however it provides useful data,
and justifies dose levels used in the teratology study which followed
(Record No. 112070). Kishiyama and Aldous, Nov. 1, 1995.
-- 031 071324 "Cryolite Animal Metabolism," (Pennwalt Corporation,
10/28/88), Summary of in-house and literature studies on cryolite.
Cryolite serves as a source of fluoride and is essentially metabolized
as free fluoride. Released in aqueous solution, the fluoride is
deposited primarily in teeth and bone. Teeth striations have been
observed in rat (3 to 13 ppm fluorine in diet) and cryolite in
new bone of rabbit mandible (12 to 50 mg fluorine/day). Osteomalacia
was produced in sheep with cryolite (60 mg fluorine/day). These
effects are in response to fluorideÕs interference with normal
calcium metabolism. Cryolite hydrolyzes in vitro to produce fluoride
anion instantaneously under acidic (pH 5: 15.5%), neutral (pH
7: 36.8%) or basic (pH 9: 43.3%) conditions. The same effect probably
also occurs in vivo, based upon the rapid assimilation into the
bone as well as itÕs efficient membrane permeability. Hydrolysis
products of cryolite may re-associate to form similar salts. Differences
in acute toxicity between cryolite and other fluoride salts is
potentially due to solubility. Inefficient absorption occurs at
levels necessary to cause acute fluoride toxicity symptoms. Chronic
effects, however, are similar to those produced by simple fluoride
salts. M. Silva, 8/22/89.
-- 031 071325 "The Comparative Toxicity of Fluorine in Calcium
Fluoride and in Cryolite," (University of Illinois, 3/29,39).
Cryolite (synthetic product), marketed as an insecticide, consisted
of 47% fluorine and calcium fluoride were fed in diet and drinking
water to albino rats (10 females and 14 males/treatment group)
at 0.58 mg/kg for 14 weeks. Several of the rats, irrespective
of treatment groups showed hematuria
lasting 1 or 2 days in the first week. Striations in tooth enamel
began to appear during the 8th week of treatment and were visible
in all rats by the end of the 10th week. Data demonstrate that
the action of fluorine from cryolite is indistinguishable from
that of calcium fluoride when both are administered in aqueous
solutions at the rate of 0.58 mg/kg daily. Approximately
96% of the fluorine retained (13 ppm in food) is deposited in
the skeleton, while the rest is equally divided between
teeth and soft tissues. M. Silva, 8/10/89.
-- 031 071330 "The Assimilation of Fluorine by Rats From Natural
and Synthetic Cryolite and From Cryolite-Sprayed Fruits," (University
of Illinois, 6/30/41). Twelve pairs or trios of rats, depending
upon the number on rations to be compared, were selected on the
basis of sex, litter membership and body weight and fed equal
amounts within the pairs or trios. Initially, 1-4 animals/litter
were sacrificed for base level of fluorine. Experiment I: Domestic
synthetic cryolite (particle size < 1 mm, 44.9% fluorine) and
natural Greenland cryolite (commercial form < 5 mm, 46.2% fluorine;
specially ground form < 1 mm, 50.5% fluorine) was fed (9.4 ppm
fluorine). Results showed that fluorine
of synthetic cryolite is retained significantly more than fluorine
from natural cryolite, probably due to solubility...M.
Silva, 8/14/89.
Ref: 1995 - Summary of Toxicology Data.
California EPA, Department of Pesticide Regulation, Medical Toxicology
Branch.
http://www.fluoridealert.org/pesticides/cryolite.ca.epa.nov.1995.pdf
Stomach
(click on for all
fluorinated pesticides)
-- 032 070618 "Cryolite:
Stomach Irritation Associated With Hydrogen Fluoride Formation,"
(Summary of scientific studies by Pennwalt). Recent studies by
Pennwalt have demonstrated stomach irritation
in rats fed cryolite. Pennwalt contends these responses are due
to fluorine rather than cryolite per se. According to Pennwalt,
clinical investigators have demonstrated that small amounts of
fluoride salts which are capable of releasing free fluoride will
produce high enough levels of HF in the stomach to result in gastric
distress (several references and examples are cited), ranging
from stomach upset and gastric ulcers in
adults to stomach hemorrhages in young children and infants.
It is known that cryolite produces free fluoride and that HF would
be produced in an acidic medium such as the stomach. Therefore,
Pennwalt requests that the stomach irritation noted in the toxicological
studies performed with cryolite be considered as relating to data
for NaF (and other salts capable of dissociation) rather than
be a basis for special concern. Pennwalt requests their cryolite
studies be considered an extension of the vast amount of fluoride
toxicology data already available. M. Silva, 8/23/89.
-- -- 145-045 139642 Nemec, M.D., "Developmental toxicity study
of Kryocide* in mice", WIL Research Laboratories, Inc. Study No.
WIL-160004, Jan. 6, 1992. Kryocide*, purity of 97.3%, was administered
via gavage at concentrations of 0 (0.5% Methylcellulose), 100,
300 or 1000 mg/kg/day to 30 mated Crl:CD-1* (ICR) BR mice/group
during gestation days 6 through 15. Maternal toxicity NOEL = 100
mg/kg/day. Mortality was 40% and 10% for high and mid dose groups,
respectively, with occasional necropsy reporting of
"red stomach contents" or "reddened adrenals"...
Ref: 1995 - Summary of Toxicology Data.
California EPA, Department of Pesticide Regulation, Medical Toxicology
Branch.
http://www.fluoridealert.org/pesticides/cryolite.ca.epa.nov.1995.pdf
|