|
Return
to Acifluorfen
Index Page
Activity:
Herbicide
(diphenyl ether)
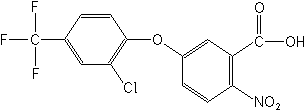
Structure for "Acifluorfen":
Adverse
Effects:
Anemia
Body Weight Decrease
Bone
Brain
Cancer: Probable Human Carcinogen - LIVER
Endocrine: Testicular
Eye
Kidney
Liver
Stomach
Environmental
•
As of February 14, 2005, this herbicide is permitted to
have residues in or on 5 food
commodities in the United States - see tolerances at bottom
of page.
|
Anemia
(click on for all fluorinated pesticides)
Increased liver and
kidney weights occurred in chronic rat, mouse, and dog studies
and were accompanied by microscopic liver and kidney changes in
the chronic rat and dog studies. Anemia
was present in chronic rat and dog studies. Stomach ulcers
were found in chronic rat and mouse studies. Testicular atrophy
occurred in the chronic rat study. Increased mortality occurred
in the high-dose group in rat and mouse studies.
Ref:
January 15, 2002. Preliminary Human Health Risk Assessment. MEMORANDUM
SUBJECT: SODIUM ACIFLUORFEN. HED Chapter for the Reregistration
Eligibility Decision Document. US EPA, Office of Prevention, Pesticides
and Toxic Substances. http://www.fluorideaction.org/pesticides/acifluorfen.na.a.red.jan.02.pdf
Body
Weight Decrease (click
on for all fluorinated pesticides)
| STUDY
TYPE – DOSE LEVELS |
NOAEL
(mg/kg/day) |
LOAEL
(mg/kg/day) |
| Chronic/Carcinogenicity
- Rat (1983) 0, 25, 150, 500, 2500, or 5000 ppm. (0, 1.25,
7.50, 25.0, 125, or 250 mg/kg/day based on 1 ppm=0.05 mg/kg/day)
(MRID No. 00128253; Accession No’s. 071315 through 071317
and 250289 through 250792) |
25 |
125
based on reduced body weight,
increased absolute and relative liver weights and increased
kidney weights, increased incidence of nephritis/pyelonephritis,
increased incidence of acidophilic cells in the liver, and
related changes in clinical chemistry parameters . |
| Carcinogenicity
in Mice (1982). 0, 625, 1250, or 2500 ppm (males: 0, 119,
259, 655 mg/kg/day; females: 0, 143, 313, 711 mg/kg/day) (MRID
No. 00122732; Accession No’s.071312, 071313, 071314, 250463,
and 250464) |
< 119 |
• • 119
. • • 143
based on reduced body weight,
increased absolute and relative liver weights, and changes
in hematologic parameters (decreased MCV counts, decreased
segmented neutrophil counts, increased RBC counts, and increased
lymphocyte counts). |
| Chronic
Feeding Study in Dogs (1983). Tackle “2S” (Acifluorfen, purity
was unspecified). 0, 20, 300, or 4500 ppm (0, 0.5, 7.5 or
112.5 mg/kg/day based on 1 ppm = 0.025 mg/kg/day) for 2 years
(MRID No. 00131162; Accession No’s.251297 and 251298) |
7.5 |
112.5
based on decreased body weight gain,
increased absolute and relative liver and kidney weights,
changes in hematology, biochemistry, and urinalysis parameters
and increased incidence of microscopic changes in the liver. |
| Developmental
Toxicity Study in Rats (1981) 0, 20, 90, or 180 mg/kg/day
from gestation days 6 through 19 (MRID No.00122743; Accession
No. 071319) |
Maternal:
20
Development al: 20 |
Maternal:
90
based on increase in clinical signs (excessive salivation
and piloerection). Developmental: 90 based on the decreased
fetal body weight and the increase in anatomical variations. |
| Developmental
Toxicity Study in Rabbits (1979) 0, 20, 60, 180 mg/kg/day
(MRID 00107485) |
Maternal:
20
Development al: 60 |
Maternal:
60
based on clinical signs (anorexia,
depression and dyspnea).
Developmental: 180 based on fetal resorptions. |
| Ref:
April 27, 2001. MEMORANDUM. SUBJECT: SODIUM ACIFLUORFEN. HED
Chapter for the Reregistration Eligibility Decision Document.
US EPA. Office of Prevention, Pesticides, and Toxic Substances.
http://www.fluorideaction.org/pesticides/acifluorfen.epa.01.healtrsk.pdf |
--
For "females 13-50 years," a NOAEL of 20 mg/kg/day
was established based on effects of decreased
fetal weight and increased incidence of dilated
lateral ventricles of the brain observed in a rat developmental
toxicity study. Both the decreased
fetal weight and the brain malformations are presumed
to occur after a single exposure (dose), and thus, are
appropriate for this acute risk assessment. These effects
were observed at 90 mg/kg/day (LOAEL).
Ref:
Overview of Sodium Acifluorfen Risk Assessment April 4,
2002. USEPA. http://www.fluorideaction.org/pesticides/acifluorfen.na.epa.apr.02.pdf
|
--
Carcinogenic Effects. Some studies show acifluorfen to
be carcinogenic, while others do not; the main differences
among the studies were the dose levels administered and
the lengths of the studies. One study of mice fed high
doses for 18 months showed decreases
in body weight and increases in both benign and
malignant liver tumors (2). As a result, acifluorfen is
classified as a probable human carcinogen by the U.S.
Environmental Protection Agency.
Ref: Pesticide Information
Profile. Extoxnet. Cornell Univeristy.
http://www.fluoridealert.org/pesticides/Acifluorfen.Extoxnet.htm
|
Bone (click
on for all fluorinated pesticides)
Developmental
toxicity in rats... A significant increase (p < 0.0001) in resorptions
in the high-dose group and significant reduction (p< 0.01) in
mean fetal weights for both the mid- and high-dose groups were
observed. A significant increase (p< 0.05
or 0.01) in anatomical variations was seen in the mid- and high-dose
groups. The variations included slightly dilated lateral
ventricles of the brain, hemorrhage in the eyeball, slight dilation
of the renal pelvis, hemorrhage in either the peritoneal cavity
or subcutaneous spaces, and minor changes
in ossification (such as incomplete ossification of supra-occipital
sternebra or thoracic centra). The NOAEL for developmental
toxicity is 20 mg/kg/day; the LOAEL for developmental toxicity
is 90 mg/kg/day based on the decreased fetal body weight and
the increase in anatomical variations.
Ref: November 10, 1999. Toxicology Chapter
for RED. MEMORANDUM SUBJECT: ACIFLUORFEN: TOXICOLOGY CHAPTER FOR
RED. http://www.epa.gov/pesticides/reregistration/acifluorfen/toxicology_chapter.pdf
Brain
(click
on for all fluorinated pesticides)
The toxicity database
is adequate for selecting toxicity endpoints for risk assessments,
although a developmental neurotoxicity study is required because
of neurotoxicity which occurred in a developmental rat study (dilated
lateral ventricles of the brain)... A developmental toxicity
study in rats found qualitative evidence of increased susceptibility
of offspring because developmental toxicity (increased resorptions,
reduced fetal weights, slightly dilated
lateral ventricles of the brain, hemorrhage in the eyeball,
slight dilation of the renal pelvis, hemorrhage in peritoneal
cavity and subcutaneous spaces, and changes in ossification) was
accompanied by minimal maternal toxicity (excess salivation and
piloerection)... the Hazard Identification Assessment Review Committee
recommended that a developmental neurotoxicity study in rats be
conducted based on neurotoxicity observed in a developmental toxicity
study in rats (increased incidence of dilated
lateral ventricles of the fetal brain, MRID 00122743).
In addition, no neurotoxicity studies are available for acifluorfen
or for structurally related compounds which might provide an understanding
on the effects of acifluorfen on the developing nervous system.
Ref: EPA, Sodium Acifluorfen. HED Chapter
for the Reregistration Eligibility Decision. April 27, 2001.
http://www.epa.gov/oppsrrd1/reregistration/acifluorfen/newrisk.pdf
--
For "females 13-50 years," a NOAEL of 20 mg/kg/day was
established based on effects of decreased fetal weight and increased
incidence of dilated lateral ventricles of the brain
observed in a rat developmental toxicity study. Both the decreased
fetal weight and
the brain malformations are presumed
to occur after a single exposure (dose), and thus, are appropriate
for this acute risk assessment. These effects were observed at
90 mg/kg/day (LOAEL).
Ref:
Overview of Sodium Acifluorfen Risk Assessment April 4, 2002.
USEPA. http://www.fluorideaction.org/pesticides/acifluorfen.na.epa.apr.02.pdf
| TABLE
3. Doses and Toxicological Endpoints Selected for Various
Exposure Scenarios |
| EXPOSURE
SCENARIO |
DOSE (mg/kg/day) |
ENDPOINT |
STUDY |
| Acute
Dietary (Female 13-50) |
NOAEL=20
UF=100
FQPA SF=10 |
Decreased
fetal weight and increased incidences
of dilated lateral ventricles of the brain
at 90 mg/kg/day |
Developmental–
rat |
|
Acute
PAD = 0.02 mg/kg/day |
| (a)
Dermal (short and intermediate-term, females 13-50) |
NOAEL=20
MOE=100
FQPA SF=10
(Females 13-50)
|
Decreased
fetal weight and increased incidences
of dilated lateral ventricles of the
brain at 90 mg/kg/day |
| (b)
Inhalation (short and intermediate-term, females 13-50) |
| (a)
= Since an oral NOAEL was selected, a dermal absorption factor
of 20% of oral absorption should be used in route-to-route
extrapolation. |
|
(b) = Since an oral NOAEL was selected, an inhalation absorption
factor of 100% of oral absorption (default value) should be
used in route-to-route extrapolation. |
| Ref:
January 15, 2002. MEMORANDUM. SUBJECT: SODIUM ACIFLUORFEN.
HED Chapter for the Reregistration Eligibility Decision Document.
US EPA. Office of Prevention, Pesticides, and Toxics Substances.
http://www.fluorideaction.org/pesticides/acifluorfen.na.a.red.jan.02.pdf |
Cancer:
Probable Human Carcinogen - LIVER
(click
on for all fluorinated pesticides)
Likely
to be Carcinogenic to Humans at High Doses. Not Likely to be Carcinogenic
to Humans at Low Doses.
Liver; B6C3F1 & CD-1 mice (M & F).
Ref: April
26, 2006.
Chemicals Evaluated for Carcinogenic Potential by the Office of
Pesticide Programs.
From: Jess Rowland, Chief Science Information Management Branch
Health Effect Division (7509C) Office of Pesticide Programs, USEPA.
http://www.fluorideaction.org/pesticides/pesticides.cancer.potential.2006.pdf
Group B2--Probable
Human Carcinogen. Reviewed 3/ 17/
88.
Ref:
March 15, 2002. List of Chemicals
Evaluated for Carcinogenic Potential. Science Information Management
Branch, Health Effects Division, Office of Pesticide Programs,
U. S. Environmental Protection Agency.
http://www.biomuncie.org/chemicals_evaluated_for_carcinog.htm
-- Acifluorfen is classified
as a Group B2 compound, i.e., the chemical
is a probable human carcinogen. Acifluorfen produced an
increased incidence of combined malignant
and benign liver tumors in two different strains of mice.
The compound also displayed positive mutagenic activity in several
non-mammalian test systems, and is structurally similar to four
other diphenyl ether herbicide compounds which caused increased
incidences of liver tumors in two different strains of mice. EPA
believes that there is sufficient evidence for listing acifluorfen
sodium salt on EPCRA section 313 pursuant to EPCRA section 313(d)(2)(B)
based on the available carcinogenicity data.
Ref.
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As
cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
There are eight diphenyl ethers that are structurally similar
to diclofop-methyl. Of the chemicals, fomesafen
sodium, haloxyfop-methyl (Verdict), oxyfluorfen, acifluorfen
sodium, nitrofen, and lactofen were reviewed in the initial
CPRC report. All of these chemicals induced
liver adenomas and carcinomas in rats and/or mice. Except
for haloxyfop-methyl, all of the other chemicals
produced positive results in at least one of the mutagenicity
assays...
May
24, 2000 - Cancer
Assessment Document. Evaluation of the
Carcinogenic Potential of Diclofop-Methyl. (Second Review). Final
Report. Cancer Assessment Review Committee, Health Effects Division,
US EPA Office of Pesticide Programs.
Note:
Except for Nitrofen, all the pesticides cited above are fluorinated.
Endocrine:
Testicular
(click
on for all fluorinated pesticides)
Increased liver and
kidney weights occurred in chronic rat, mouse, and dog studies
and were accompanied by microscopic liver and kidney changes in
the chronic rat and dog studies. Anemia was present in chronic
rat and dog studies. Stomach ulcers were found in chronic rat
and mouse studies. Testicular atrophy occurred
in the chronic rat study. Increased mortality occurred
in the high-dose group in rat and mouse studies.
Ref:
January 15, 2002. Preliminary Human Health Risk Assessment. MEMORANDUM
SUBJECT: SODIUM ACIFLUORFEN. HED Chapter for the Reregistration
Eligibility Decision Document. US EPA, Office of Prevention, Pesticides
and Toxic Substances. http://www.fluorideaction.org/pesticides/acifluorfen.na.a.red.jan.02.pdf
Eye
(click on for all fluorinated
pesticides)
Acute Toxicity: Sodium
acifluorfen has been placed in Acute Toxicity
Category I for acute eye irritation and in Category II
for acute dermal irritation.
Ref:
April 4, 2002. Overview of Sodium Acifluorfen Risk Assessment.
USEPA.
http://www.fluorideaction.org/pesticides/acifluorfen.na.epa.apr.02.pdf
A developmental
toxicity study in rats found qualitative evidence of increased
susceptibility of offspring because developmental toxicity (increased
resorptions, reduced fetal weights, slightly dilated lateral ventricles
of the brain, hemorrhage in the eyeball,
slight dilation of the renal pelvis, hemorrhage in peritoneal
cavity and subcutaneous spaces, and changes in ossification)
Ref: January 15, 2002. MEMORANDUM. SUBJECT:
SODIUM ACIFLUORFEN. HED Chapter for the Reregistration Eligibility
Decision Document. http://www.fluorideaction.org/pesticides/acifluorfen.na.a.red.jan.02.pdf
Severe
eye irritant;
moderate skin irritant (rabbit). /Acifluorfen/ [Tomlin, C.D.S.
(ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey,
UK: The British Crop Protection Council, 1994]
Ref: TOXNET profile from Hazardous Substances
Data Base for ACIFLUORFEN-SODIUM..
http://www.fluoridealert.org/pesticides/Acifluorfen.NA.TOXNET.HSDB.htm
Acifluorfen is very
toxic and is labeled with a DANGER signal word due to its potential
to cause serious eye injury.
Ref: Pesticide Information Profile. Extoxnet
from Cornell Univeristy.
http://www.fluoridealert.org/pesticides/Acifluorfen.Extoxnet.htm
Kidney
(click
on for all fluorinated pesticides)
A 2-generation reproduction
study in rats fed diets containing 0, 25, 500 or 2,500 ppm with
no adverse effect on adult reproductive performance observed under
the conditions of the study. The NOEL was established at 25 ppm
(equivalent to 1.25 mg/kg of body weight/day) based on decreased
viability and increased incidence of kidney
lesions in high dose offspring.
Ref: Federal Register. April 17, 1996. Sodium
Salt of Acifluorfen; Pesticide Tolerance. Proposed Rule.
http://www.fluoridealert.org/pesticides/Acifluorfen.Sod.FR.Apr17.96.htm
The chronic
feeding toxicity study in rats, mice, and dogs demonstrated that
acifluorfen induced liver toxicity (acidophilic cells in the liver
and increased liver weight) and kidney toxicity
(nephritis/pyelonephritis and increased kidney weight).
An increase in the incidence of stomach ulcer was also seen in
chronic feeding study in rats.
Ref: US EPA Toxicology Chapter for RED.
November 10, 1999
http://www.epa.gov/pesticides/reregistration/acifluorfen/toxicology_chapter.pdf
Liver
(click
on for all fluorinated pesticides)
Carcinogenicity.
Acifluorfen has been classified as a Group B2 (probable human
carcinogen) chemical by the OPP Cancer Peer Review Committee (CPRC),
based on an increased number of liver tumors in both sexes of
mice and a high incidence of uncommonly
occurring stomach papillomas in male mice. The
Committee recommended using the Q1* approach for quantification
of human risk. The Q1* is 0.11 (mg/kg/day)-1.
Ref: Federal Register. July 25, 1997. Sodium
Salt of Acifluorfen; Pesticide Tolerances for Emergency Exemptions.
Final Rule.
http://www.epa.gov/fedrgstr/EPA-PEST/1997/July/Day-25/p19668.htm
Male
mice had a significant increasing trend,
and significant differences in the pair-wise comparisons of all
dose groups with the controls, for liver
adenoma and/or carcinoma tumors combined.
Ref: MEMORANDUM. November 8, 2001. SUBJECT:
REVISED Sodium Salt of Aciflourfen (Tackle TM , Blazer TM ) Quantitative
Risk Assessment (Q1 * ) Based On B6C3F1 Mouse Dietary Study Using
mg/kg b.w.^ 3 /4 's/day Cross Species Scaling Factor.
http://www.fluorideaction.org/pesticides/acifluorfen.na.cancer.nov01.pdf
Stomach
(click
on for all fluorinated pesticides)
Increased liver and kidney weights occurred in chronic rat, mouse,
and dog studies and were accompanied by microscopic liver and
kidney changes in the chronic rat
and dog studies. Anemia was present in chronic rat and dog studies.
Stomach ulcers were found in chronic rat and mouse studies.
Testicular atrophy occurred in the chronic rat study. Increased
mortality occurred in the high-dose group in rat and mouse studies.
Ref:
January 15, 2002. Preliminary Human Health Risk Assessment. MEMORANDUM
SUBJECT: SODIUM
ACIFLUORFEN. HED Chapter for the Reregistration Eligibility Decision
Document. US EPA, Office of Prevention, Pesticides and Toxic Substances.
http://www.fluorideaction.org/pesticides/acifluorfen.na.a.red.jan.02.pdf
| Environmental:
(click on for all fluorinated
pesticides)
Persistent
in soil and water; Phototoxic.
--
Ecological Risk: ... The Agency (USEPA) is uncertain about
risks to freshwater and estuarine animals. The acute toxicity
data do not suggest a risk concern. However, EPA does not
have sufficient information to assess chronic risk. A no
observed adverse effect level could not be determined in
a chronic fish toxicity study because the lowest dose level
resulted in an effect (reduced larvae weight). A comparison
of the maximum peak concentration of acifluorfen in water
is 100 fold lower than the LC50 for rainbow trout or bluegill
sunfish. Because acifluorfen is persistent in water, the
Agency is concerned about the potential for chronic risk.
EPA is also concerned about the potential for chronic risk
based on the phototoxic mechanism of action of sodium acifluorfen.
Confirmatory data will be required to address this concern.
Ref: Overview of Sodium
Acifluorfen Risk Assessment April 4, 2002. USEPA. http://www.fluorideaction.org/pesticides/acifluorfen.na.epa.apr.02.pdf
--
Environmental Fate: Sodium acifluorfen is extremely water
soluble, is stable to hydrolysis and is moderately
persistent to persistent in soil and water. Aerobic
soil metabolism half-lives ranged from 30 days up to 6 months;
anaerobic soil metabolism half-life was less than 28 days.
Acifluorfen is very mobile with low binding potential. In
soil (pH > 3.5), acifluorfen is predominately an anion with
little sorption in many soils. Acifluorfen binding increases
with soil organic carbon content. Soil temperature and soil
water content influence soil microbial activity and may
influence acifluorfen's degradation rate. The decarboxy
derivative of acifluorfen was the primary degradate found
in solution. The amino analog of acifluorfen (amino acifluorfen)
is the major degradate under anaerobic soil conditions.
Depending upon soil type, amino acifluorfen ranged from
immobile to medium mobility. The aerobic aquatic half-life
was estimated to be 117 days.
-- In ground water, acifluorfen will
be persistent due to its stability to abiotic hydrolysis.
During runoff events, sodium acifluorfen may reach surface
waters from ground water where it would also persist for
some time (unless there is some photodegradation; <1 to
29 days half-life). Acifluorfen would not be expected to
bioaccumulate in fish because of the low Kow value.
Ref: January 15, 2002. Preliminary
Human Health Risk Assessment. MEMORANDUM SUBJECT: SODIUM
ACIFLUORFEN. HED Chapter for the Reregistration Eligibility
Decision Document. US EPA, Office of Prevention, Pesticides
and Toxic Substances. http://www.fluorideaction.org/pesticides/acifluorfen.na.a.red.jan.02.pdf
|
A
February 14, 2005, check at the Code
of Federal Regulations for the sodium salt of Acifluorfen:
this herbicide is permitted in
or on 5 food commodities. The
following list identifies these crops for which EPA has
set pesticide tolerances.
Note:
see
Tolerance Actions published in the Federal
Register on February 11, 2004.
|
| [Code
of Federal Regulations]
[Title 40, Volume 22]
[Revised as of July 1, 2004]
From the U.S. Government Printing Office via GPO Access
[CITE: 40CFR180.383]
[Page 427]
TITLE 40--PROTECTION OF ENVIRONMENT
CHAPTER I--ENVIRONMENTAL PROTECTION AGENCY (CONTINUED)
PART 180_TOLERANCES AND EXEMPTIONS FROM TOLERANCES FOR PESTICIDE
CHEMICALS
IN FOOD--Table of Contents
Subpart C_Specific Tolerances
Sec. 180.383 Sodium salt of acifluorfen; tolerances for residues.
(a) General. Tolerances are established
for combined residues of the
herbicide sodium salt of acifluorfen (sodium 5-[2-chloro-4-tri
fluoro
methyl) phen oxy]-2-nitrobenzoic acid) and
its metabolites (the
corresponding acid, methyl ester, and amino analogues)
in or on the
following raw agricultural commodities: |
| Commodity |
As
of
September 13,
2003
PPM |
As
of
February 14,
2005
PPM |
CFR |
| Peanut |
0.1 |
0.1 |
180.383
|
| Rice,
grain |
0.1 |
0.1 |
180.383
|
| Rice,
straw |
0.1 |
0.1 |
180.383
|
Soybean
|
0.1 |
0.1 |
180.383
|
| Strawberry |
0.05 |
0.05
|
180.383
|
| CATTLE,
KIDNEY |
0.02 |
Not
listed |
180.383
|
| CATTLE,
LIVER |
0.02 |
Not
listed |
180.383
|
| EGG |
0.02 |
Not
listed |
180.383
|
| GOAT, KIDNEY |
0.02 |
Not
listed |
180.383
|
| GOAT, LIVER |
0.02 |
Not
listed |
180.383
|
| HOG, KIDNEY |
0.02 |
Not
listed |
180.383
|
| HOG, LIVER |
0.02 |
Not
listed |
180.383
|
| HORSE,
KIDNEY |
0.02 |
Not
listed |
180.383
|
| HORSE,
LIVER |
0.02 |
Not
listed |
180.383
|
| MILK |
0.02 |
Not
listed |
180.383
|
| POULTRY,
FAT |
0.02 |
Not
listed |
180.383
|
| POULTRY,
MEAT |
0.02 |
Not
listed |
180.383
|
| POULTRY,
MEAT BYPRODUCTS |
0.02 |
Not
listed |
180.383
|
| SHEEP,
KIDNEY |
0.02 |
Not
listed |
180.383
|
| SHEEP,
LIVER |
0.02 |
Not
listed |
180.383
|
| SOYBEAN |
0.1 |
Not
listed |
180.383
|
| STRAWBERRY |
0.05 |
Not
listed |
180.383
|
| CATTLE,
KIDNEY |
0.02 |
Not
listed |
180.383
|
| CATTLE,
LIVER |
0.02 |
Not
listed |
180.383
|
| EGG |
0.02 |
Not
listed |
180.383
|
| GOAT, KIDNEY |
0.02 |
Not
listed |
180.383
|
| GOAT, LIVER |
0.02 |
Not
listed |
180.383
|
| HOG, KIDNEY |
0.02 |
Not
listed |
180.383
|
| HOG, LIVER |
0.02 |
Not
listed |
180.383
|
| HORSE,
KIDNEY |
0.02 |
Not
listed |
180.383
|
| HORSE,
LIVER |
0.02 |
Not
listed |
180.383
|
| MILK |
0.02 |
Not
listed |
180.383
|
| PEANUT |
0.1 |
Not
listed |
180.383
|
| POULTRY,
FAT |
0.02 |
Not
listed |
180.383
|
| POULTRY,
MEAT |
0.02 |
Not
listed |
180.383
|
| POULTRY,
MEAT BYPRODUCTS |
0.02 |
Not
listed |
180.383
|
| RICE, GRAIN |
0.1 |
Not
listed |
180.383
|
| RICE, STRAW |
0.1 |
Not
listed |
180.383
|
| SHEEP,
KIDNEY |
0.02 |
Not
listed |
180.383
|
| SHEEP,
LIVER |
0.02 |
Not
listed |
180.383
|
| (b)
Section 18 emergency exemptions. [Reserved]
(c) Tolerances with regional restrictions. [Reserved]
(d) Indirect or inadvertent residues. [Reserved] |
|

