|
Return to
Adverse Effects
Abstracts
ACTIVITY: Herbicide
(diphenyl ether)
Scientific Name: SODIUM 5-(2-CHLORO-4-(TRIFLUOROMETHYL)PHENOXY)-2-NITROBENZOATE
Also known as:
• 5-(2-chloro-4- (triflouromethyl)phenoxy)-2-nitro-benzoic
acid, sodium salt
• BENZOIC ACID, 5-(2-CHLORO-4-(TRIFLUOROMETHYL)PHENOXY)-2-NITRO-,
SODIUM SALT
Structure
for Acifluorfen:
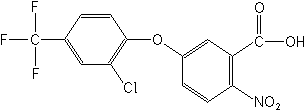
| Adverse
Effects:
Anemia
Body Weight Decrease
Bone
Brain
Cancer: Probable Human Carcinogen -
LIVER
Endocrine: Testicular
Eye
Kidney
Liver
Stomach |
| Environmental
Effects:
Persistent
in soil and water.
Phototoxic |
The
following Regulatory Information
(only comprehensive for the US) |
| US
EPA Registered: |
Yes
|
| US
EPA PC Code: |
114402
|
| Old
US EPA PC Code |
20980
|
| California
Chemical Code |
2218 |
| US
Tolerances: |
CFR
180.383 |
Acifluorfen:
Registered
use in
|
Canada |
Acifluorfen, sodium:
Registered
use in |
Australia,
Hungary, Italy (soybean), US |
| European Commission: |
Acifluorfen:
Not
allowed to be used as an active ingredient after July 25, 2003.
However,
a specific exemption for continued
use of Acifluorfen has been allowed for
use on Soybean in Italy
|
US
Maximum Residue Tolerance Levels
|
Permitted
in or on 5 food commodities in the US:
Peanut;
Rice, grain; Rice, straw; Soybean, seed; Strawberry |
| Other
Information |
| Molecular
Formula: |
C14H7Cl
F3 NO5 ´Na |
| Inventing
Co.: |
Mobil |
EPA
Permit Date
and Registrant: |
1980,
Rohm & Haas |
| Manufacturers: |
Aventis
(Rhone-Poulenc),
Rohm & Haas,
BASF sold to United
Phosphorus Ltd. in 2003 |
| Other
Names: |
Blazer
2L
Blazer 2S
Blazer
Carbofuorfen
Status
Tackle 2AS
MC-10978
RH-6201 |
| Manufacture
site: |
US:
Rohm & Haas, Laporte, Texas 77571 |
| Of special interest: |
| PAN
BAD ACTOR - Carcinogen |
| Classified
by the State of California: "Known to Cause Cancer" |
| 2006. Re-evaluation
of Acifluorfen. PACR2006-02. Canada Pest Management Regulatory
Agency. |
| US
EPA RED Assessment |
| US
Map of 1991-1995 Pesticide Use |
| US
Map of 1995-1998 Pesticide Use |
| US
Herbicide Products |
| US
Toxic Release Inventory. Year: 2000 |
| Profile
from Hazardous Substances Data Bank |
Sept.
26, 2003: BASF
sells acifluorfen to United Phosphorus
BASF HAS sold the compound acifluorfen to United Phosphorus
Ltd., the companies have jointly announced. Acifluorfen, a herbicide
active ingredient, is used primarily in the United States and
Brazil in such products as Ultra Blazer, Storm and Volt herbicides.
The sale includes all registrations, brands and trademarks.
The sale is not subject to approval by any regulatory bodies.
United Phosphorus, Inc., the North American subsidiary of UPL,
will begin selling Ultra Blazer and Storm immediately in the
United States. I shortly thereafter 2004,, UPL will begin selling
Ultra Blazer and related acifluorfen products elsewhere in the
world. |
| April 4, 2002 -
Summary
of EPA's human health and ecological risk findings for the herbicide
sodium acifluorfen |
| Feb
11, 2002 -
EPA's response to
BASF's comments concerning the Environmental Fate, Transport
and Water Assessment. |
| Jan
15, 2002 -
Revised human health
risk assessment for reregistration of the herbicide, sodium
acifluorfen - US EPA |
| Dec
18, 2001 - Revised
Product and Residue Chemistry Chapters of the Reregistration
Eligibility Decision (RED) - US EPA |
| Dec
18, 2001 - BASF
Comments Regarding the Product and Residue Chemistry Chapters
of the RED document |
| Dec
6, 2001 -
Dietary Exposure Analyses
for the HED Preliminary Human Health Risk Assessment -
US EPA |
| Dec
3, 2001 -
EPA's response to
BASF comments to the Occupational and Non-Occupational Exposure
and Risk |
| Nov
29, 2001 -
EPA's response to
BASF's Phase 3 Comments on the Preliminary Risk Assessment Document
for Sodium Acifluorfen. |
| Nov
13, 2001 - Second
Revised Occupational and Residential Exposure and Risk Assessment
for the Reregistration Eligibility Decision -
US EPA |
| Nov
8, 2001 -
Revised Quantitative
Risk Assessment (Tackle , Blazer) -
US EPA |
| July
2001 - Documents
Available on Tolerance Reassessment and Reregistration -
US EPA |
| June
7, 2001 - Overview
of Sodium Acifluorfen Risk Assessment -
US EPA |
| April
27, 2001 -
Revised Human Health
Risk Assessment for the reregistration of sodium acifluorfen
- US EPA |
| March
7, 2001 - Phototoxic
Pesticides: US EPA Memo Requesting
Phototoxicity Study Protocol for Light-Dependent Peroxidizing
Herbicides |
May
24, 2000 -
Cancer Assessment Document.
Evaluation of the Carcinogenic Potential of Diclofop-Methyl.
(Second Review). Final Report.
Cancer Assessment Review Committee, Health Effects Division,
US EPA Office of Pesticide Programs. "There are eight diphenyl
ethers that are structurally similar to diclofop-methyl. Of
the chemicals, fomesafen sodium,
haloxyfop-methyl (Verdict), oxyfluorfen,
acifluorfen sodium, nitrofen,
and lactofen were
reviewed in the initial CPRC report. All of these chemicals
induced liver adenomas and carcinomas in rats and/or mice..."
Note from FAN: the
pesticides highlighted in red are organofluorines |
| June
8, 2000 -
Ecological Risk and
Drinking Water Assessment - US EPA
|
| April
21, 1994 -
Acifluorfen:
Toxicology summary data - California
EPA |
| See also Acifluorfen
(CAS No. 50594-66-6) |
| Abstracts |
| Note:
Acifluorfen is normally used as a salt
or an ester, the identity of which should be stated, e.g. acifluorfen-sodium
[62476-59-9]. Two esters have their
own common names: fluoroglycofen
and lactofen. |
| Rationale
for US EPA to add Acifluorfen, sodium
to the Toxic Release Inventory
Acifluorfen
sodium salt
(5-(2-chloro-4- (triflouromethyl)phenoxy)-2-nitro-benzoic
acid, sodium salt). Acifluorfen is classified as a Group B2
compound, i.e., the chemical is a probable
human carcinogen. Acifluorfen produced an increased
incidence of combined malignant and benign liver tumors in
two different strains of mice. The compound also displayed
positive mutagenic activity in several non-mammalian test
systems, and is structurally similar to four other diphenyl
ether herbicide compounds which caused increased incidences
of liver tumors in two different strains of mice. EPA believes
that there is sufficient evidence for listing acifluorfen
sodium salt on EPCRA section 313 pursuant to EPCRA section
313(d)(2)(B) based on the available carcinogenicity data.
Ref.
USEPA/OPP. Support Document for the Addition of Chemicals
from Federal Insecticide, Fungicide, Rodenticide Act (FIFRA)
Active Ingredients to EPCRA Section 313. U. S. Environmental
Protection Agency, Washington, DC (1993). As
cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372.
Addition of Certain Chemicals; Toxic Chemical Release Reporting;
Community Right-to-Know; Proposed Rule. |
| US
MAPS. Estimated Annual Agricultural
Use
as compiled by the Pesticide National Synthesis Project of
the U. S. Geological Survey's National Water Quality Assessment
Program |
| Crops |
From:
1991-1995
Data Map
Total Pounds Applied |
From:
1995-1998
Data Map
Total Pounds Applied |
From:
1991-1995
Data Map
Percent National Use |
From:
1995-1999
Data Map
Percent National Use |
| Soybeans |
1,517,072 |
1,705,603 |
89.69 |
92.46 |
| Peanuts |
148,196 |
112,526 |
8.76 |
6.10 |
| Rice |
26,284 |
25,516 |
1.55 |
1.44 |
| November
26, 2002 |
Acifluorfen
-
European Commission: one of 320
pesticides to be withdrawn in July 2003.
However, a specific exemption for continued use of Acifluorfen
has been allowed for use on Soybean in Italy. "Some
320 substances used in plant protection products (PPPs) -
including insecticides, fungicides and herbicides Ð are to
be withdrawn from the market by 25 July 2003 as part of the
European Commission's new approach to the evaluation of active
substances in plant protection products. This aims to improve
safeguards to ensure that all such products in use are safe
for the environment and human health. Users, wholesalers and
retailers of plant protection products will need to be aware
of whether the products they use or sell are likely to be
withdrawn, so as to prevent them being left with stocks of
unusable material. Those concerned should contact their national
authority to check the authorisation status for any particular
product. The Regulation (n¡ 2076/2002 of 20 November 2002),
with the list of the 320 substances, has been published in
the Offical Journal.
Ref: MIDDAY EXPRESS. News from the Press and Communication
Service's midday briefing. |
| Dec 10,
2001 |
Australia:
Current List. RECORD
OF APPROVED ACTIVE CONSTITUENTS FOR CHEMICAL PRODUCTS. |
| Oct 2001 |
Glossary
of Pesticide Chemicals. A listing of pesticides subject
to analysis of residues in foods and feeds by the US Food and
Drug Administration. |
| August
8, 2001 |
Material
Safety Data Sheet, Restek Corporation. The herbicide product
[Name: 32061, 32061-5XX, & 32161 / Herbicide Mix # 4 / (Free
acid)], contains 0.02 % Acifluorfen. |
| July 25,
2001 |
Registerred
in the European Union (EU) countries: France, Italy. EU Note:
"Out 7/03" -
Ref: "DOCUMENT
SANCO/2692/2001 OF 25 JULY 2001 WORKING DOCUMENT OF THE COMMISSION
SERVICES TECHNICAL ANNEX TO REPORT FROM THE COMMISSION TO THE
EUROPEAN PARLIAMENT AND THE COUNCIL ON THE EVALUATION OF THE
ACTIVE SUBSTANCES OF PLANT PROTECTION PRODUCTS." |
| April
2000 |
Tobacco
Engineered for Resistance to the Herbicide Acifluorfen -
Information Systems for Biotechnology |
| US EPA |
Index
of Cleared Science Reviews |
| Cornell
Extoxnet |
Pesticide Information Profile |
| Environmental
Defense Fund |
Chemical
Profile
List
of Pesticides which contain Acifluorfen |
| Australia |
December
10, 2001 - "Current
List. RECORD OF APPROVED ACTIVE CONSTITUENTS FOR CHEMICAL PRODUCTS."
|
| California |
California
Air Toxics "Hot Spots" List (Assembly Bill 2588) |
| US
Federal Register |
| Published
Date |
Docket
Identification Number |
Details
|
| September 15, 2006 |
EPA-HQ-OPP-2005-0459 |
Tolerance
Actions. FINAL
RULE.
EPA is modifying certain tolerances for the herbicide sodium
acifluorfen. The regulatory actions in this document are part
of the Agency's reregistration program under the FIFRA.
Tolerances are established for combined residues of the herbicide
sodium salt of acifluorfen, sodium 5-[2-chloro-4-
(trifluoromethyl)phenoxy]-2-nitrobenzoate, and its metabolites
(the corresponding acid, methyl ester, and amino analogues)
in or on the following raw agricultural commodities:
-- Peanut at 0.1 ppm
-- Rice, grain at 0.1 ppm
-- Rice, straw at 0.2 ppm
-- Soybean, seed at 0.1 ppm
-- Strawberry at 0.05 ppm
Peanut;
Rice, grain; Rice, straw; Soybean, seed; Strawberry.
|
| April 26, 2006 |
EPA-HQ-OPP-2005-0459 |
Sodium
Acifluorfen and Trifluralin; Proposed Tolerance Actions.
EPA is proposing to modify certain tolerances for the herbicides
sodium acifluorfen and trifluralin. Electronic copies of REDs
and TREDs are available on the internet for endosulfan, fenarimol,
imazalil, oryzalin, sodium acifluorfen, trifluralin, and ziram
in public dockets EPA-HQ-OPP-2003-0293, and EPA-HQ-OPP-2004-0142,
respectively, at http://www.regulations.gov. |
| Feb
22, 2006 |
EPA-HQ-OPP-2006-0084 |
Requests
to Voluntarily Cancel Certain Pesticide Registrations.
Unless a request is withdrawn by August 21, 2006, orders will
be issued canceling these registrations. The Agency will consider
withdrawal requests postmarked no later than August 21, 2006.
|
Registration no. |
Product
Name |
Chemical
Name |
Registrants
Requesting Voluntary Cancellation |
| 007969-00080 |
Blazer
2S Herbicide |
Benzoic
acid, 5-(2-chloro-4-(trifluoromethyl)phenoxy)-2-nitro,
sodium salt |
BASF
Corp., Agricultural
Products, PO Box 13528,
Research Triangle Pa, NC 27709-3528. |
|
007969-00168 |
Conclude
Ultra Herbicide |
same |
|
|
007969-00179 |
Conclude
Xact |
same |
same |
| 007969-00077
|
Galaxy
Herbicide |
same |
same |
| 000241-00321
|
Scepter
O.T. Herbicide |
same |
same |
|
| August
17, 2005 |
OPP-2005-0222 |
Requests
to Voluntarily Cancel Certain Pesticide Registrations.
|
Registration no. |
Product
Name |
Chemical
Name |
Registrants
Requesting Voluntary Cancellation |
|
007969-00168 |
Conclude
Ultra Herbicide |
Benzoic
acid, 5-(2-chloro-4-(trifluoromethyl)phenoxy)-2-nitro-,
sodium salt |
BASF
Corp., Agricultural
Products, PO Box 13528,
Research Triangle Pa, NC 27709-3528. |
|
007969-00179 |
Conclude
Xact |
same |
same |
| 007969-00077
|
Galaxy
Herbicide |
same |
same |
| 007969-00080
|
Blazer
2S Herbicide |
same |
same |
| 000241-00321
|
Scepter
O.T. Herbicide |
same |
BASF
Corp., PO Box 13528,
Research Triangle Pa, NC 27709-3528. |
|
| Feb
11, 2004 |
OPP-2003-0344 |
Tolerance
Actions. FINAL
RULE. This final
rule revokes specific meat, milk, poultry, and egg (MMPE)
tolerances for residues of the insecticide sodium acifluorfen.
EPA
determined that there are no reasonable expectations of finite
residues in or on meat, milk, poultry, or eggs for the aforementioned
pesticide active
ingredients and that these tolerances are no longer needed.
The regulatory
actions in this document contribute toward the Agency's tolerance
reassessment requirements of the Federal Food, Drug, and Cosmetic
Act (FFDCA) section 408(q), as amended by the Food Quality
Protection Act (FQPA) of 1996. By law, EPA is required by
August 2006 to reassess the tolerances in existence on August
2, 1996. Because all the tolerances were previously reassessed,
no reassessments are counted here toward the August, 2006
review deadline.
-- Label
restrictions prohibit use of sodium acifluorfen treated peanut
and soybean forage or hay for feed and grazing livestock on
these treated crops. As noted in a memorandum dated April
21, 2002, available under docket ID number OPP-2003-0092,
EPA evaluated available ruminant and poultry metabolism data
and determined that there is no reasonable expectation of
residues being transferred to livestock commodities via consumption
of feed items derived from crops treated with sodium acifluorfen
according to current use directions. Based on the registered
food/feed use patterns and metabolism data, EPA determined
that there is no reasonable expectation of finite residues
of sodium acifluorfen and its metabolites in eggs;
kidney and liver of cattle, goats, hogs, horses, and sheep;
fat, meat, and meat byproducts of poultry; and milk. These
tolerances are no longer needed under 40 CFR 180.6(a)(3).
Therefore, EPA is revoking the tolerances
in 40 CFR 180.383 for combined residues of the herbicide sodium
salt of acifluorfen (sodium 5-[2-chloro-4-trifluoromethyl)
phenoxy]-2-nitrobenzoic acid) and
its metabolites (the corresponding acid, methyl ester, and
amino analogues) in or on the following commodities:
Cattle, kidney; cattle, liver; egg; goat, kidney; goat, liver;
hog, kidney; hog, liver; horse, kidney; horse, liver; milk;
poultry, fat; poultry, meat; poultry, meat byproducts; sheep,
kidney; and sheep, liver. |
| Jan 28, 2004 |
OPP-2003-293 |
Reregistration
Eligibility Decision document (RED);
Notice of Availability.
For the herbicide sodium
acifluorfen, EPA is announcing the availability of the RED
document and supporting technical documents. EPA has assessed
the risks
associated with the use of sodium acifluorfen, reassessed
the tolerances for sodium acifluorfen, and reached a reregistration
eligibility decision. The Agency has determined that all currently
registered uses of sodium acifluorfen are eligible for reregistration,
provided that all the conditions identified in the RED document
are
satisfied, including the implementation of risk mitigation
measures through label amendments. The RED document also describes
the tolerance reassessment decision for sodium acifluorfen.
Comments on the RED must be
received by EPA on or before February 27, 2004. |
| July
30, 2003 |
OPP-2002-0327 |
US
EPA's Pesticide Reregistration Performance Measures and Goals.
Sodium
acifluorfen: Candidate for Reregistration Eligibility Decision
(RED) in Fiscal Year 2004
which
runs
from October 1, 2003, through September 30, 2004. |
| Sept
13, 2002 |
OPP-2002-
0121 |
EPA
status of reregistration and tolerance reassessment. |
| May
17, 2002 |
OPP-2002-0037 |
Revocation
of Expired Time Limited Tolerances. FINAL RULE. Time-limited
tolerances for cowpeas, lima beans, and Southern peas are being
removed from 40 CFR 180.383 because they expired on December
31, 1998. |
| April
12, 2002 |
OPP-2002-0008 |
Availability
of Revised Risk Assessments (Blazer). The documents being
released to the public through this notice provide some new
information on the human health effects of sodium acifluorfen,
and information on the revisions that were made to the sodium
acifluorfen preliminary risk assessments, which were released
to the public July 26, 2001 (66 FR 3904) (FRL-6789-4), through
a notice in the Federal Register. This notice starts a 45-day
public participation period during which the public is encouraged
to submit comments on the new information not available previously
during the earlier public comment period for the sodium acifluorfen
preliminary risk assessment, and risk management proposals or
other comments on risk management for sodium acifluorfen. |
|
August 4, 1997 |
OPP-300523 |
Pesticides
Subject to Tolerance Reassessment. |
| July
25, 1997 |
OPP-300516 |
Pesticide
Tolerances for Emergency Exemptions. - FINAL RULE.
This regulation establishes time-limited tolerances for the
combined residues of the sodium salt of acifluorfen and its
metabolites in or on lima beans, cowpeas, and southern peas
at 0.1 PPM.. The tolerances will expire and are revoked on December
31, 1998. |
| June
14, 1996 |
PP
0E3821/R2242 |
Pesticide
Tolerance for strawberry at 0.05 ppm.- FINAL RULE. |
| April
17, 1996 |
PP
0E3821/P649 |
Proposed
Pesticide Tolerance for strawberry at 0.05 ppm. |
| Jan
12, 1994 |
OPPTS-400082 |
EPA's
proposal to add 41
fluorine and organofluorine chemicals to the Toxics Release
Inventory (TRI). See excerpt in box
above. Also available at http://www.epa.gov/tri/frnotices/59fr1788.htm |
| 1982 |
na |
From
TOXNET: Acifluorfen,
sodium: TOLERANCES AND EXEMPTIONS FROM TOLERANCES FOR PESTICIDE
CHEMICALS IN OR ON RAW AGRICULTURAL COMMODITIES - 47:39490 |
|