|
Return to Index Page
Molecular
structure of Novaluron:
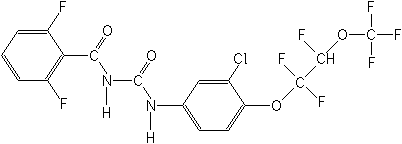
http://www.epa.gov/fedrgstr/EPA-PEST/2004/June/Day-02/p12316.htm
[Federal Register: June 2, 2004 (Volume 69, Number 106)]
[Rules and Regulations]
[Page 31013-31022]
From the Federal Register Online via GPO Access [wais.access.gpo.gov]
[DOCID:fr02jn04-9]
-----------------------------------------------------------------------
ENVIRONMENTAL PROTECTION AGENCY
40 CFR Part 180
[OPP-2004-0125; FRL-7359-2]
Novaluron; Pesticide Tolerance
AGENCY: Environmental Protection Agency (EPA).
ACTION: Final rule.
-----------------------------------------------------------------------
SUMMARY: This regulation establishes tolerances for residues of
novaluron in or on fruit, pome (group 11), apple, wet pomace;
cotton,
undelinted seed; cotton, gin byproducts; vegetables, tuberous
and corm,
subgroup 1C; meat, fat, and meat byproducts of sheep, horse, cattle,
goat, hog, and poultry; milk; milk, fat; and eggs. Makhteshim-Agan
of
North America, Inc. requested this tolerance under the Federal
Food,
Drug, and Cosmetic Act (FFDCA), as amended by the Food Quality
Protection Act of 1996 (FQPA).
DATES: This regulation is effective June 2, 2004. Objections
and
requests for hearings must be received on or before August 2,
2004.
ADDRESSES: To submit a written objection or hearing request follow
the
detailed instructions as provided in Unit VI. of the SUPPLEMENTARY
INFORMATION. EPA has established a docket for this action under
Docket
ID number OPP-2004-0125. All documents in the docket are listed
in the
EDOCKET index at kenny.dan@epa.gov.
SUPPLEMENTARY INFORMATION:
I. General Information
A. Does this Action Apply to Me?
You may be potentially affected by this action if you are an
agricultural producer, food manufacturer, or pesticide manufacturer.
Potentially affected entities may include, but are not limited
to:
• Crop production (NAICS 111), e.g., agricultural workers;
greenhouse, nursery, and floriculture workers; farmers.
[[Page 31014]]
• Animal production (NAICS 112), e.g., cattle ranchers and
farmers, dairy cattle farmers, livestock farmers.
• Food manufacturing (NAICS 311), e.g., agricultural
workers; farmers; greenhouse, nursery, and floriculture workers;
ranchers; pesticide applicators.
• Pesticide manufacturing (NAICS 32532), e.g., agricultural
workers; commercial applicators; farmers; greenhouse, nursery,
and
floriculture workers; residential users.
This listing is not intended to be exhaustive, but rather provides
a guide for readers regarding entities likely to be affected by
this
action. Other types of entities not listed in this unit could
also be
affected. The North American Industrial Classification System
(NAICS)
codes have been provided to assist you and others in determining
whether this action might apply to certain entities. If you have
any
questions regarding the applicability of this action to a particular
entity, consult the person listed under FOR FURTHER INFORMATION
CONTACT.
B. How Can I Access Electronic Copies of this Document and Other
Related Information?
In addition to using EDOCKET (http://www.epa.gov/edocket/), you
may
access this Federal Register document electronically through the
EPA
Internet under the ``Federal Register'' listings at http://www.epa.gov/
fedrgstr/. A frequently updated electronic version of 40 CFR part
180
is available at E-CFR Beta Site Two at http://www.gpoaccess.gov/ecfr/.II.
Background and Statutory Findings
In the Federal Register of February 25, 2004 (69 FR 8649) (FRL-
7344-6), EPA issued a notice pursuant to section 408(d)(3) of
FFDCA, 21
U.S.C. 346a(d)(3), announcing the filing of a pesticide petition
(PP
2F6430) by Makhteshim-Agan of North America,
Inc., 551 Fifth Avenue,
Suite 1100, New York, NY 10176. That notice included a
summary of the
petition prepared by Makhteshim-Agan of North America, Inc., the
registrant. There were no comments received in response to the
notice
of filing.
The petition requested that 40 CFR 180 be amended by establishing
tolerances for residues of the insecticide
novaluron, 1-[3-chloro-4-
(1,1,2-trifluoro-2-trifluoro-methoxyethoxy)phenyl]-3-(2,6-
difluorobenzoyl)urea, in or on fruit, pome (group 11) at 2.0 parts
per
million (ppm), apple, wet pomace at 8.0 ppm; cotton, undelinted
seed at
0.60 ppm; cotton, gin byproducts at 30 ppm; vegetables, tuberous
and
corm, subgroup 1C at 0.05 ppm; sheep, horse, cattle, and goat,
meat at
0.60 ppm; sheep, horse, cattle, and goat, meat byproducts (except
liver
and kidney) at 0.60 ppm; sheep, horse, cattle, and goat, fat at
11 ppm;
sheep, horse, cattle, and goat, liver at 1.0 ppm; sheep, horse,
cattle,
and goat, kidney at 1.0 ppm; milk at 1.0 ppm; milk, fat at 20
ppm; hog,
meat at 0.01 ppm; hog, meat byproducts at 0.01 ppm; hog, fat at
0.05
ppm; poultry, meat at 0.03 ppm; poultry, meat byproducts at 0.04
ppm;
poultry, fat at 0.40 ppm; and eggs at 0.05 ppm.
Section 408(b)(2)(A)(i) of FFDCA allows EPA to establish a
tolerance (the legal limit for a pesticide chemical residue in
or on a
food) only if EPA determines that the tolerance is ``safe.'' Section
408(b)(2)(A)(ii) of FFDCA defines ``safe'' to mean that ``there
is a
reasonable certainty that no harm will result from aggregate exposure
to the pesticide chemical residue, including all anticipated dietary
exposures and all other exposures for which there is reliable
information.'' This includes exposure through drinking water and
in
residential settings, but does not include occupational exposure.
Section 408(b)(2)(C) of FFDCA requires EPA to give special
consideration to exposure of infants and children to the pesticide
chemical residue in establishing a tolerance and to ``ensure that
there
is a reasonable certainty that no harm will result to infants
and
children from aggregate exposure to the pesticide chemical residue.
. .
.''
EPA performs a number of analyses to determine the risks from
aggregate exposure to pesticide residues. For further discussion
of the
regulatory requirements of section 408 of FFDCA and a complete
description of the risk assessment process, see the final rule
on
Bifenthrin Pesticide Tolerances (62 FR 62961, November 26, 1997)
(FRL-
5754-7).
III. Aggregate Risk Assessment and Determination of Safety
Consistent with section 408(b)(2)(D) of FFDCA, EPA has reviewed
the
available scientific data and other relevant information in support
of
this action. EPA has sufficient data to assess the hazards of
and to
make a determination on aggregate exposure, consistent with section
408(b)(2) of FFDCA, for tolerances for residues of novaluron on
fruit,
pome (group 11) at 2.0 ppm, apple, wet pomace at 8.0 ppm; cotton,
undelinted seed at 0.60 ppm; cotton, gin byproducts at 30 ppm;
vegetables, tuberous and corm, subgroup 1C at 0.05 ppm; sheep,
horse,
cattle, and goat, meat at 0.60 ppm; sheep, horse, cattle, and
goat,
meat byproducts (except liver and kidney) at 0.60 ppm; sheep,
horse,
cattle, and goat, fat at 11 ppm; sheep, horse, cattle, and goat,
liver
at 1.0 ppm; sheep, horse, cattle, and goat, kidney at 1.0 ppm;
milk at
1.0 ppm; milk, fat at 20 ppm; hog, meat at 0.01 ppm; hog, meat
byproducts at 0.01 ppm; hog, fat at 0.05 ppm; poultry, meat at
0.03
ppm; poultry, meat byproducts at 0.04 ppm; poultry, fat at 0.40
ppm;
and eggs at 0.05 ppm. EPA's assessment of exposures and risks
associated with establishing the tolerance follows.
A. Toxicological Profile
EPA has evaluated the available toxicity data and considered
its
validity, completeness, and reliability as well as the relationship
of
the results of the studies to human risk. EPA has also considered
available information concerning the variability of the sensitivities
of major identifiable subgroups of consumers, including infants
and
children. The nature of the toxic effects caused by novaluron
are
discussed in Table 1 of this unit as well as the no observed adverse
effect level (NOAEL) and the lowest observed adverse effect level
(LOAEL) from the toxicity studies reviewed.
| Table
1.--Subchronic, Chronic, and Other Toxicity |
| Guideline
No |
Study |
Type Results |
| 870.3200
|
28-day
Dermal toxicity - rat |
Systemic
NOAEL= 1,000 mg/kg/day;
LOAEL= not established
Dermal NOAEL= 1,000 mg/kg/day;
LOAEL= not established |
| 870.3700 |
Prenatal
Developmental in rodents-rat. |
Maternal
NOAEL: >=1,000; LOAEL: not established
Developmental NOAEL: >= 1,000; LOAEL: not established |
[[Page
31015]]
870.3700
|
Prenatal
developmental in nonrodents- rabbit. |
Maternal
NOAEL: >=1,000; LOAEL: not established
Developmental NOAEL: >= 1,000; LOAEL: not established |
| 870.3800
|
Reproduction
and fertility-
rat |
Parental
NOAEL= Not established; LOAEL (M/F)= 74.2/84.0 mg/kg/day
based on increased absolute and relative
spleen weights.
Offspring NOAEL= Not established;
LOAEL (M/F)= 74.2/84.0 mg/ kg/day based on increased
absolute and relative spleen weights.
Reproductive NOAEL (M/F)= 74.2
>=1009.8 mg/kg/day;
LOAEL= 297.5 mg/kg/ day based on decreased
epididymal sperm counts and increased age of preputial separation
in the F1 generation, reproductive LOAEL for females was not
established |
| 870.4100
|
Chronic
toxicity - dog |
NOAEL=
10 mg/kg/day
LOAEL=100 mg/kg/day
based on hematologic changes associated
with histopathological changes in liver and spleen |
| 870.4300
|
Chronic/
carcinogenicity- rat |
NOAEL (M/F)
=1.1/1.4 mg/kg/day
LOAEL (M/F)=30.6/39.5 mg/kg/day
based on Erythrocyte damage and turnover
resulting in a regenerative mild anemia |
| 870.4300 |
Chronic/
carcinogenicity- mouse |
NOAEL (M/F)=3.6/4.3
mg/kg/day
LOAEL (M/F)=53.4/63.3 mg/kg/day
based on increased erythrocyte turnover
due to hemoglobin oxidation and resulting in a mild anemia |
| 870.5100
|
Salmonella
typhimurium and Escherichia coli - Reverse Mutation Assay. |
Novaluron,
tested up to the limit of solubility (2,500 [mu]g/plate) and
the limit dose (5,000 [mu]g/plate), was not cytotoxic with or
without S9 activation in four S. typhimurium strains and one
strain of E. coli, and did not induce a genotoxic response in
any strain |
| 870.5100 |
Salmonella
typhimurium - bacterial reverse gene mutation assay. |
Novaluron,
tested up to the limit of solubility (3333 [mu]g/plate), was
not cytotoxic with or without S9 activation in five S. typhimurium
strains, and did not induce a genotoxic response in any strain |
| 870.5300
|
Gene mutation
|
There was
no evidence of biologically significant induction of mutant
colonies over background |
| 870.5375 |
In vitro
mammalian chromosome aberration test. |
Novaluron
produced no evidence of clastogenic activity in primary human
lymphocytes, in the presence or absence of S9 activation |
| 870.5395
|
Mammalian
erythrocyte micronucleus test in mice. |
There was
no statistically significant increase
in the frequency of micronucleated polychromatic erythrocytes
in mouse bone marrow at any dose or harvest time |
| 870.5550
|
Unscheduled
DNA Synthesis in HeLa S3 Human Epitheliod cells. |
Novaluron
was considered not to show any
evidence of causing DNA damage to HeLa S3 epithelioid cells
in this unscheduled DNA synthesis test for mutagenic potential |
| 870.5500
|
Mutagenicity-rec
assay with Bacillus subtilis. |
Novaluron
was equivocal for bacterial DNA
damage in the absence of S9 activation, and negative for bacterial
DNA damage in the presence of S9 activation |
| 870.6200
|
Acute neurotoxicity
screening battery- rat. |
NOAEL=
650 mg/kg/ day; LOAEL=2,000 mg/ kg/day based on clinical signs
(piloerection, irregular breathing), FOB parameters (increased
head swaying, abnormal gait) and neuropathology
(sciatic and tibial nerve degeneration). |
| 870.6200
|
Subchronic
neurotoxicity screening battery- rat. |
NOAEL (M/F)=>=1,752/
>=2,000 mg/kg/day;
LOAEL= not established |
| 870.7485
|
Metabolism-rat
|
Novaluron
exhibited marginal absorption (16-18%), relatively rapid and
complete excretion within 48 hours primarily via the feces and
to a lesser extent via urine in rat |
| 870.7600
|
Rat Dermal
penetration |
|
| M
- Male; F - Female |
[[Page 31016]]
B. Toxicological Endpoints
The dose at which the NOAEL from the toxicology study identified
as
appropriate for use in risk assessment is used to estimate the
toxicological level of concern (LOC). However, the LOAEL is sometimes
used for risk assessment if no NOAEL was achieved in the toxicology
study selected. An uncertainty factor (UF) is applied to reflect
uncertainties inherent in the extrapolation from laboratory animal
data
to humans and in the variations in sensitivity among members of
the
human population as well as other unknowns. An UF of 100 is routinely
used, 10X to account for interspecies differences and 10X for
intraspecies differences.
Three other types of safety or uncertainty factors may be used:
``Traditional uncertainty factors;'' the ``special FQPA safety
factor;'' and the ``default FQPA safety factor.'' By the term
``traditional uncertainty factor,'' EPA is referring to those
additional uncertainty factors used prior to FQPA passage to account
for database deficiencies. These traditional uncertainty factors
have
been incorporated by the FQPA into the additional safety factor
for the
protection of infants and children. The term ``special FQPA safety
factor'' refers to those safety factors that are deemed necessary
for
the protection of infants and children primarily as a result of
the
FQPA. The ``default FQPA safety factor'' is the additional 10X safety
factor that is mandated by the statute unless it is decided that
there
are reliable data to choose a different additional factor (potentially
a traditional uncertainty factor or a special FQPA safety factor).
For dietary risk assessment (other than cancer) the Agency uses
the
UF to calculate an acute or chronic reference dose (acute RfD or
chronic RfD) where the RfD is equal to the NOAEL divided by an UF
of
100 to account for interspecies and intraspecies differences and
any
traditional uncertainty factors deemed appropriate (RfD = NOAEL/UF).
Where a special FQPA safety factor or the default FQPA safety factor
is
used, this additional factor is applied to the RfD by dividing the
RfD
by such additional factor. The acute or chronic Population Adjusted
Dose (aPAD or cPAD) is a modification of the RfD to accommodate
this
type of safety factor.
For non-dietary risk assessments (other than cancer) the UF is used
to determine the LOC. For example, when 100 is the appropriate UF
(10X
to account for interspecies differences and 10X for intraspecies
differences) the LOC is 100. To estimate risk, a ratio of the NOAEL
to
exposures (margin of exposure (MOE) = NOAEL/exposure) is calculated
and
compared to the LOC.
The linear default risk methodology (Q*) is the primary method
currently used by the Agency to quantify carcinogenic risk. The
Q*
approach assumes that any amount of exposure will lead to some degree
of cancer risk. A Q* is calculated and used to estimate risk which
represents a probability of occurrence of additional cancer cases
(e.g., risk). An example of how such a probability risk is expressed
would be to describe the risk as one in one hundred thousand (1
x
10-5), one in a million (1 x 106), or one in ten
million (1 x 107). Under certain specific circumstances, MOE
calculations will be used for the carcinogenic risk assessment.
In this
non-linear approach, a ``point of departure'' is identified below
which
carcinogenic effects are not expected. The point of departure is
typically a NOAEL based on an endpoint related to cancer effects
though
it may be a different value derived from the dose response curve.
To
estimate risk, a ratio of the point of departure to exposure
(MOEcancer = point of departure/exposures) is calculated.
A summary of the toxicological endpoints for novaluron used for
human risk assessment is shown in Table 2 of this unit:
| Table
2.--Summary of Toxicological Dose and Endpoints for novaluron
for Use in Human Risk Assessment |
| Exposure
Scenario |
Dose Used
in Risk
Assessment, Interspecies and Intraspecies and any Traditional
UF |
Special
FQPA SF* and
LOC for Risk Assessment |
Study and
Toxicological Effects |
| Acute dietary
|
Not applicable |
None |
An endpoint
of concern attributable to a single dose was not identified.
An acute RfD was not established |
| Chronic
dietary (All populations) |
NOAEL=
1.1 mg/kg/day
UF = 100...............
Chronic RfD = 0.011 mg/kg/day. |
FQPA SF
= 1X cPAD = chronic RfD/FQPA SF = 0.011 mg/kg/day...... |
Combined
chronic toxicity/ carcinogenicity feeding in rat LOAEL = 30.6
mg/kg/day based on erythrocyte damage
and turnover resulting in a regenerative anemia |
| Short-term
incidental oral (1-30 days) |
NOAEL=
4.38 mg/kg/day |
Residential
LOC for MOE = 100
Occupational LOC for MOE = 100. |
90-day
feeding study in rat
LOAEL = 8.64 mg/kg/day
based on clinical chemistry (decreased
hemoglobin, hematocrit and RBC counts) and histopathology (increased
hematopoiesis and hemosiderosis in spleen and liver). |
| Intermediate-term
incidental oral (1- 6 months) |
NOAEL=
4.38 mg/kg/day |
Residential
LOC for MOE = 100
Occupational LOC for MOE = 100. |
90-day
feeding study in rat
LOAEL = 8.64 mg/kg/day
based on clinical chemistry (decreased
hemoglobin, hematocrit and RBC counts) and histopathology (increased
hematopoiesis and hemosiderosis in spleen and liver) |
| Short-term
dermal (1 to 30 days) |
Not applicable |
None |
No toxicity
observed at the limit dose in dermal study and there were no
developmental toxicity concerns at the limit-dose; therefore,
quantification of short-term dermal risk is not necessary |
[[Page
31017]]
Intermediate-term
dermal (1 to 6 months) |
Oral NOAEL
= 4.38 mg/kg/ day(dermal-absorption rate = 10%) |
Residential
LOC for MOE = 100
Occupational LOC for MOE = 100. |
90-day
feeding study in rat
LOAEL = 8.64 mg/kg/day
based on clinical chemistry (decreased
hemoglobin, hematocrit and RBC counts) and histopathology (increased
hematopoiesis and hemosiderosis in spleen and liver) |
| Long-term
dermal (>6months) |
Oral NOAEL=
1.1 mg/kg/ day (dermal-absorption rate = 10 %) |
Residential
LOC for MOE = 100
Occupational LOC for MOE = 100. |
Combined
chronic toxicity/ carcinogenicity feeding in rat
LOAEL = 30.6 mg/kg/day
based on erythrocyte damage and turnover
resulting in a regenerative anemia |
| Short-term
inhalation (1 to 30 days) |
Oral NOAEL
= 4.38 mg/kg/ day (inhalation absorption rate = 100%) |
Residential
LOC for MOE = 100
Occupational LOC for MOE = 100. |
90-day feeding study in rat
LOAEL = 8.64 mg/kg/day
based on clinical chemistry (decreased
hemoglobin, hematocrit and RBC counts) and histopathology (increased
hematopoiesis and hemosiderosis in spleen and liver) |
| Intermediate-term
inhalation (1 to 6 months) |
Oral NOAEL
= 4.38 mg/kg/ day (inhalation absorption rate = 100%) |
Residential
LOC for MOE = 100
Occupational LOC for MOE = 100. |
90-day
feeding study in rat
LOAEL = 8.64 mg/kg/day
based on clinical chemistry (decreased
hemoglobin, hematocrit and RBC counts) and histopathology (increased
hematopoiesis and hemosiderosis in spleen and liver).
|
| Long-term
inhalation (>6 months) |
Oral NOAEL=
1.1 mg/kg/ day (inhalation absorption rate = 100%) |
Residential
LOC for MOE = 100
Occupational LOC for MOE = 100. |
Combined
chronic toxicity/ carcinogenicity feeding in rat
LOAEL = 30.6 mg/kg/day
based on erythrocyte damage and turnover
resulting in a regenerative anemia |
| Cancer |
Not
likely to be carcinogenic to humans |
C. Exposure Assessment
1. Dietary exposure from food
and feed uses. Currently, no
tolerances have been established for the residues of novaluron,
in or
on any raw agricultural commodities. Risk assessments were
conducted by
EPA to assess dietary exposures from novaluron in food as follows:
i. Acute exposure. Acute dietary risk assessments are performed
for
a food-use pesticide, if a toxicological study has indicated the
possibility of an effect of concern occurring as a result of a 1-day
or
single exposure. An endpoint of concern attributable to a single
dose
of novaluron was not identified. Therefore, an acute dietary risk
assessment was not conducted.
ii. Chronic exposure. In conducting the chronic dietary
risk
assessment EPA used the Dietary Exposure Evaluation Model software
with
the Food Commodity Intake Database (DEEM-FCID\TM\), which incorporates
food consumption data as reported by respondents in the USDA 1994-1996
and 1998 nationwide Continuing Surveys of Food Intake by Individuals
(CSFII), and accumulated exposure to the chemical for each commodity.
The following assumptions were made for the chronic exposure
assessments: The chronic analysis assumed 100% crop treated for
all
commodities; incorporated average field trial residues; empirical
processing factors for apple juice (translated to pear juice); and
DEEM\TM\ (ver 7.76) default processing factors for the remaining
processed commodities. Anticipated residues were calculated for
meat
and milk commodities and recommended tolerances were used for poultry
commodities.
iii. Cancer. Novaluron is classified
as ``not likely to be
carcinogenic to humans'' based on the lack of evidence for
carcinogenicity in mice and rats. Therefore, a quantitative cancer
risk
assessment was not conducted.
iv. Anticipated residue and percent crop treated (PCT)
information.
Section 408(b)(2)(E) of FFDCA authorizes EPA to use available data
and
information on the anticipated residue levels of pesticide residues
in
food and the actual levels of pesticide chemicals that have been
measured in food. If EPA relies on such information, EPA must require
that data be provided 5 years after the tolerance is established,
modified, or left in effect, demonstrating that the levels in food
are
not above the levels anticipated. Following the initial data
submission, EPA is authorized to require similar data on a time
frame
it deems appropriate. As required by section 408(b)(2)(E) of FFDCA,
EPA
will issue a data call-in for information relating to anticipated
residues to be submitted no later than 5 years from the date of
issuance of this tolerance.
2. Dietary exposure from drinking water. The Agency
lacks
sufficient monitoring exposure data to complete a comprehensive
dietary
exposure analysis and risk assessment for novaluron in drinking
water.
Because the Agency does not have comprehensive monitoring data,
drinking water concentration estimates are made by reliance on
simulation or modeling taking into account data on
[[Page 31018]]
the physical characteristics of novaluron. Novaluron
may reach surface
water or ground water via the parent compound or via its chlorophenyl
urea and chloroaniline degradates. Therefore, concentrations of
novaluron and its chlorophenyl urea and chloroaniline degradates
in
surface water and ground water were estimated by using modeling.
The Agency uses the Generic Estimated Environmental Concentration
(GENEEC) or the Pesticide Root Zone Model/Exposure Analysis Modeling
System (PRZM/EXAMS) to estimate pesticide concentrations in surface
water and screening concentration in ground water (SCI-GROW), which
predicts pesticide concentrations in ground water. In general, EPA
will
use GENEEC (a Tier I model) before using PRZM/EXAMS (a Tier II model)
for a screening-level assessment for surface water. The GENEEC model
is
a subset of the PRZM/EXAMS model that uses a specific high-end runoff
scenario for pesticides. GENEEC incorporates a farm pond scenario,
while PRZM/EXAMS incorporate an index reservoir environment in place
of
the previous pond scenario. The PRZM/EXAMS model includes a percent
crop area factor as an adjustment to account for the maximum percent
crop coverage within a watershed or drainage basin. Tier II Pesticide
Root Zone Model/Exposure Analysis Modeling System (PRZM/EXAMS) modeling
was performed to estimate drinking water concentrations for novaluron
(parent) in surface water.
The Agency uses the FQPA Index Reservoir Screening Tool (FIRST)
or
the Pesticide Root Zone Model/Exposure Analysis Modeling System
(PRZM/
EXAMS), to produce estimates of pesticide concentrations in an index
reservoir. The SCI-GROW model is used to predict pesticide
concentrations in shallow ground water. For a screening-level
assessment for surface water EPA will use FIRST (a Tier I model)
before
using PRZM/EXAMS (a Tier II model). The FIRST model is a subset
of the
PRZM/EXAMS model that uses a specific high-end runoff scenario for
pesticides. Both FIRST and PRZM/EXAMS incorporate an index reservoir
environment, and both models include a percent crop area factor
as an
adjustment to account for the maximum percent crop coverage within
a
watershed or drainage basin. The FIRST model was used to obtain
surface
water estimates for the degradate chlorophenyl urea and chloroaniline.
The estimated drinking water concentration values are meant to
represent upper-bound estimates of the concentrations that might
be
found in surface water and ground water based upon existing and
proposed uses. Of the three estimated drinking water concentration
values, chronic estimates for the terminal metabolite, chloroaniline
are the highest (100% conversion from parent to aniline was assumed).
This is consistent with the expected degradation pattern for novaluron.
Therefore, the estimated drinking water concentration value for
chloroaniline was used to assess chronic aggregate risk.
None of these models include consideration of the impact processing
(mixing, dilution, or treatment) of raw water for distribution as
drinking water would likely have on the removal of pesticides from
the
source water. The primary use of these models by the Agency at this
stage is to provide a screen for sorting out pesticides for which
it is
unlikely that drinking water concentrations would exceed human health
LOC.
Since the models used are considered to be screening tools in the
risk assessment process, the Agency does not use estimated
environmental concentrations (EECs), which are the model estimates
of a
pesticide's concentration in water. EECs derived from these models
are
used to quantify drinking water exposure and risk as a %RfD or %PAD.
Instead drinking water levels of comparison (DWLOCs) are calculated
and
used as a point of comparison against the model estimates of a
pesticide's concentration in water. DWLOCs are theoretical upper
limits
on a pesticide's concentration in drinking water in light of total
aggregate exposure to a pesticide in food, and from residential
uses.
Since DWLOCs address total aggregate exposure to novaluron they
are
further discussed in the aggregate risk sections in this Unit.
Based on the PRZM/EXAMS, FIRST and SCI-GROW models, the EECs of
novaluron for chronic exposures are estimated to be 2.61 parts per
billion (ppb) for surface water and 0.009 ppb for ground water.
Since
an acute dietary risk assessment was not needed, EECs of novaluron
for
acute exposures to surface water and ground water were not used.
3. From non-dietary exposure. The term ``residential exposure''
is
used in this document to refer to non-occupational, non-dietary
exposure (e.g., for lawn and garden pest control, indoor pest control,
termiticides, and flea and tick control on pets). Novaluron is not
registered for use on any sites that would result in residential
exposure.
4. Cumulative effects from substances with a common mechanism of
toxicity. Section 408(b)(2)(D)(v) of FFDCA requires that, when
considering whether to establish, modify, or revoke a tolerance,
the
Agency consider ``available information'' concerning the cumulative
effects of a particular pesticide's residues and ``other substances
that have a common mechanism of toxicity.''
Unlike other pesticides for which EPA has followed a cumulative
risk approach based on a common mechanism of toxicity, EPA has not
made
a common mechanism of toxicity finding as to novaluron and any other
substances and novaluron does not appear to produce a toxic metabolite
produced by other substances. For the purposes of this tolerance
action, therefore, EPA has not assumed that novaluron has a common
mechanism of toxicity with other substances. For information regarding
EPA's efforts to determine which chemicals have a common mechanism
of
toxicity and to evaluate the cumulative effects of such chemicals,
see
the policy statements released by EPA's Office of Pesticide Programs
concerning common mechanism determinations and procedures for
cumulating effects from substances found to have a common mechanism
on
EPA's web site at http://www.epa.gov/pesticides/cumulative/.
D. Safety Factor for Infants and Children
1. In general. Section 408 of FFDCA provides that
EPA shall apply
an additional tenfold margin of safety for infants and children
in the
case of threshold effects to account for prenatal and postnatal
toxicity and the completeness of the data base on toxicity and exposure
unless EPA determines based on reliable data that a different margin
of
safety will be safe for infants and children. Margins of safety
are
incorporated into EPA risk assessments either directly through use
of a
MOE analysis or through using uncertainty (safety) factors in
calculating a dose level that poses no appreciable risk to humans.
In
applying this provision, EPA either retains the default value of
10X
when reliable data do not support the choice of a different factor,
or,
if reliable data are available, EPA uses a different additional
safety
factor value based on the use of traditional uncertainty factors
and/or
special FQPA safety factors, as appropriate.
2. Prenatal and postnatal sensitivity. There are no
residual
uncertainties for pre-/post-natal toxicity. There is no quantitative
or
qualitative evidence of increased susceptibility of rat and rabbit
fetuses to in utero exposure to novaluron in developmental toxicity
[[Page 31019]]
studies. There is no quantitative or qualitative evidence
of increased
susceptibility to novaluron following pre-/post-natal exposure in
a 2-
generation reproduction study.
3. Conclusion. There is a complete toxicity data base
for novaluron
and exposure data are complete or are estimated based on data that
reasonably accounts for potential exposures.
The FQPA SF was reduced to
1X, based upon the following: As mentioned above, there is
no
quantitative or qualitative evidence of increased susceptibility
of rat
and rabbit fetuses to in utero exposure to novaluron in developmental
toxicity studies. There is no quantitative or qualitative evidence
of
increased susceptibility to novaluron following pre-/post-natal
exposure in a 2-generation reproduction study. In addition, there
is no
concern for developmental neurotoxicity resulting from exposure
to
novaluron, and a developmental neurotoxicity study (DNT) study is
not
required. Furthermore, the chronic dietary food exposure assessment
assumes 100% crops treated for all commodities. The dietary drinking
water assessment utilizes water concentration values generated by
model
and associated modeling parameters which are designed to provide
conservative, health protective, high-end estimates of water
concentrations which will not likely be exceeded. Finally, there
are no
proposed or existing uses for novaluron which result in residential
exposure.
E. Aggregate Risks and Determination of Safety
To estimate total aggregate exposure to a pesticide from food,
drinking water, and residential uses, the Agency calculates DWLOCs
which are used as a point of comparison against EECs. DWLOC values
are
not regulatory standards for drinking water. DWLOCs are theoretical
upper limits on a pesticide's concentration in drinking water in
light
of total aggregate exposure to a pesticide in food and residential
uses. In calculating a DWLOC, the Agency determines how much of
the
acceptable exposure (i.e., the PAD) is available for exposure through
drinking water (e.g., allowable chronic water exposure (mg/kg/day)
=
cPAD - (average food + residential exposure)). This allowable exposure
through drinking water is used to calculate a DWLOC.
A DWLOC will vary depending on the toxic endpoint, drinking water
consumption, and body weights. Default body weights and consumption
values as used by the EPA's Office of Water are used to calculate
DWLOCs: 2 liter (L)/70 kg (adult male), 2L/60 kg (adult female),
and
1L/10 kg (child). Default body weights and drinking water consumption
values vary on an individual basis. This variation will be taken
into
account in more refined screening-level and quantitative drinking
water
exposure assessments. Different populations will have different
DWLOCs.
Generally, a DWLOC is calculated for each type of risk assessment
used:
Acute, short-term, intermediate-term, chronic, and cancer.
When EECs for surface water and ground water are less than the
calculated DWLOCs, EPA concludes with reasonable certainty that
exposures to the pesticide in drinking water (when considered along
with other sources of exposure for which EPA has reliable data)
would
not result in unacceptable levels of aggregate human health risk
at
this time. Because EPA considers the aggregate risk resulting from
multiple exposure pathways associated with a pesticide's uses, levels
of comparison in drinking water may vary as those uses change. If
new
uses are added in the future, EPA will reassess the potential impacts
of residues of the pesticide in drinking water as a part of the
aggregate risk assessment process.
1. Acute risk. An endpoint of concern attributable
to a single dose
was not identified. Therefore, no acute risk is expected.
2. Chronic risk. Using the exposure assumptions described
in this
unit for chronic exposure, EPA has concluded that exposure to novaluron
from food will utilize 18% of the cPAD for the U.S. population,
68% of
the cPAD for children 1 to 2 years old. There are no residential
uses
for novaluron that result in chronic residential exposure to novaluron.
In addition, there is potential for chronic dietary exposure to
novaluron in drinking water. After calculating DWLOCs and comparing
them to the EECs for surface and ground water, EPA does not expect
the
aggregate exposure to exceed 100% of the cPAD, as shown in Table
3 of
this unit:
| Table
3.--Aggregate Risk Assessment for Chronic (Non-Cancer) Exposure
to Novaluron |
| Population
Subgroup |
cPAD
mg/kg/ |
%
cPAD
(Food) |
Surface
Water EEC
(ppb) |
Ground
Water EEC
(ppb) |
Chronic
DWLOC
(ppb) |
| U.S. population
|
0.011 |
18 |
2.61 |
0.009
|
320 |
| Females,
(13-49 years old) |
0.011 |
12 |
2.61 |
0.009
|
290 |
| All infants |
0.011 |
31 |
2.61 |
0.009
|
76 |
| Children,
(1-2 years old) |
0.011 |
68 |
2.61 |
0.009
|
35 |
| Youth,
(13-19 years) |
0.011 |
16 |
2.61 |
0.009
|
280 |
3. Short-term risk. Short-term aggregate exposure takes into
account residential exposure plus chronic exposure to food and water
(considered to be a background exposure level). Novaluron is not
registered for use on any sites that would result in residential
exposure. Therefore, the aggregate risk is the sum of the risk from
food and water, which do not exceed the Agency's LOC.
4. Intermediate-term risk. Intermediate-term aggregate
exposure
takes into account residential exposure plus chronic exposure to
food
and water (considered to be a background exposure level). Novaluron
is
not registered for use on any sites that would result in residential
exposure. Therefore, the aggregate risk is the sum of the risk from
food and water, which do not exceed the Agency's LOC.
5. Aggregate cancer risk for U.S. population. Novaluron
has not
been shown to be carcinogenic. Therefore, novaluron is not expected
to
pose a cancer risk.
6. Determination of safety. Based on these risk assessments,
EPA
concludes that there is a reasonable certainty that no harm will
result
to the general
[[Page 31020]]
population, and to infants and children from aggregate
exposure to
novaluron residues.
IV. Other Considerations
A. Analytical Enforcement Methodology
Adequate enforcement methodology -- is available to enforce the
tolerance expression. The method may be requested from: Chief,
Analytical Chemistry Branch, Environmental Science Center, 701 Mapes
Rd., Ft. Meade, MD 20755-5350; telephone number: (410) 305-2905;
e-mail
address: residuemethods@epa.gov.
B. International Residue Limits
There are currently no established Codex, Canadian, or Mexican
maximum residue limits for novaluron.
V. Conclusion
Therefore, the tolerances are established for residues
of
novaluron, 1-[3-chloro-4-(1,1,2-trifluoro-2-trifluoro-
methoxyethoxy)phenyl]-3-(2,6-difluorobenzoyl)urea, in or on fruit,
pome
(group 11) at 2.0 ppm, apple, wet pomace at 8.0 ppm; cotton, undelinted
seed at 0.60 ppm; cotton, gin byproducts at 30 ppm; vegetables,
tuberous and corm, subgroup 1C at 0.05 ppm; sheep, horse, cattle,
and
goat, meat at 0.60 ppm; sheep, horse, cattle, and goat, meat byproducts
(except liver and kidney) at 0.60 ppm; sheep, horse, cattle, and
goat,
fat at 11 ppm; sheep, horse, cattle, and goat, liver at 1.0 ppm;
sheep,
horse, cattle, and goat, kidney at 1.0 ppm; milk at 1.0 ppm; milk,
fat
at 20 ppm; hog, meat at 0.01 ppm; hog, meat byproducts at 0.01 ppm;
hog, fat at 0.05 ppm; poultry, meat at 0.03 ppm; poultry, meat
byproducts at 0.04 ppm; poultry, fat at 0.40 ppm; and eggs at 0.05
ppm.
VI. Objections and Hearing Requests
Under section 408(g) of FFDCA, as amended by FQPA, any person may
file an objection to any aspect of this regulation and may also
request
a hearing on those objections. The EPA procedural regulations which
govern the submission of objections and requests for hearings appear
in
40 CFR part 178. Although the procedures in those regulations require
some modification to reflect the amendments made to FFDCA by FQPA,
EPA
will continue to use those procedures, with appropriate adjustments,
until the necessary modifications can be made. The new section 408(g)
of FFDCA provides essentially the same process for persons to
``object'' to a regulation for an exemption from the requirement
of a
tolerance issued by EPA under new section 408(d) of FFDCA, as was
provided in the old sections 408 and 409 of FFDCA. However, the
period
for filing objections is now 60 days, rather than 30 days.
A. What Do I Need to Do to File an Objection or Request a Hearing?
You must file your objection or request a hearing on this
regulation in accordance with the instructions provided in this
unit
and in 40 CFR part 178. To ensure proper receipt by EPA, you must
identify docket ID number OPP-2004-0125 in the subject line on the
first page of your submission. All requests must be in writing,
and
must be mailed or delivered to the Hearing Clerk on or before August
2,
2004.
1. Filing the request. Your objection must specify
the specific
provisions in the regulation that you object to, and the grounds
for
the objections (40 CFR 178.25). If a hearing is requested, the
objections must include a statement of the factual issues(s) on
which a
hearing is requested, the requestor's contentions on such issues,
and a
summary of any evidence relied upon by the objector (40 CFR 178.27).
Information submitted in connection with an objection or hearing
request may be claimed confidential by marking any part or all of
that
information as CBI. Information so marked will not be disclosed
except
in accordance with procedures set forth in 40 CFR part 2. A copy
of the
information that does not contain CBI must be submitted for inclusion
in the public record. Information not marked confidential may be
disclosed publicly by EPA without prior notice.
Mail your written request to: Office of the Hearing Clerk (1900C),
Environmental Protection Agency, 1200 Pennsylvania Ave., NW.,
Washington, DC 20460-0001. You may also deliver your request to
the
Office of the Hearing Clerk in Rm.104, Crystal Mall #2, 1921
Jefferson Davis Hwy., Arlington, VA. The Office of the Hearing Clerk
is
open from 8 a.m. to 4 p.m., Monday through Friday, excluding legal
holidays. The telephone number for the Office of the Hearing Clerk
is
(703) 603-0061.
2. Tolerance fee payment. If you file an objection
or request a
hearing, you must also pay the fee prescribed by 40 CFR 180.33(i)
or
request a waiver of that fee pursuant to 40 CFR 180.33(m). You must
mail the fee to: EPA Headquarters Accounting Operations Branch,
Office
of Pesticide Programs, P.O. Box 360277M, Pittsburgh, PA 15251. Please
identify the fee submission by labeling it ``Tolerance Petition
Fees.''
EPA is authorized to waive any fee requirement ``when in the
judgement of the Administrator such a waiver or refund is equitable
and
not contrary to the purpose of this subsection.'' For additional
information regarding the waiver of these fees, you may contact
James
Tompkins by phone at (703) 305-5697, by e-mail at tompkins.jim@epa.gov,
or by mailing a request for information to Mr. Tompkins at Registration
Division (7505C), Office of Pesticide Programs, Environmental
Protection Agency, 1200 Pennsylvania Ave., NW., Washington, DC 20460-
0001.
If you would like to request a waiver of the tolerance objection
fees, you must mail your request for such a waiver to: James Hollins,
Information Resources and Services Division (7502C), Office of
Pesticide Programs, Environmental Protection Agency, 1200 Pennsylvania
Ave., NW., Washington, DC 20460-0001.
3. Copies for the Docket. In addition to filing an objection or
hearing request with the Hearing Clerk as described in Unit VI.A.,
you
should also send a copy of your request to the PIRIB for its inclusion
in the official record that is described in Unit I.B.1. Mail your
copies, identified by docket ID number
OPP-2004-0125, to: Public
Information and Records Integrity Branch, Information Resources
and
Services Division (7502C), Office of Pesticide Programs, Environmental
Protection Agency, 1200 Pennsylvania Ave., NW., Washington, DC 20460-
0001. In person or by courier, bring a copy to the location of the
PIRIB described in Unit I.B.1. You may also send an electronic copy
of
your request via e-mail to: opp-docket@epa.gov. Please use an ASCII
file format and avoid the use of special characters and any form
of
encryption. Copies of electronic objections and hearing requests
will
also be accepted on disks in WordPerfect 6.1/8.0 or ASCII file format.
Do not include any CBI in your electronic copy. You may also submit
an
electronic copy of your request at many Federal Depository Libraries.
B. When Will the Agency Grant a Request for a Hearing?
A request for a hearing will be granted if the Administrator
determines that the material submitted shows the following: There
is a
genuine and substantial issue of fact; there is a reasonable
possibility that available evidence identified by the requestor
would,
if established resolve one or more of such issues in favor of the
requestor, taking into account uncontested claims or facts to the
contrary; and resolution of the factual issues(s) in the manner
sought
by the requestor would be adequate to justify the action requested
(40
CFR 178.32).
[[Page 31021]]
VII. Statutory and Executive Order Reviews
This final rule establishes a tolerance under section 408(d) of
FFDCA in response to a petition submitted to the Agency. The Office
of
Management and Budget (OMB) has exempted these types of actions
from
review under Executive Order 12866, entitled Regulatory Planning
and
Review (58 FR 51735, October 4, 1993). Because this rule has been
exempted from review under Executive Order 12866 due to its lack
of
significance, this rule is not subject to Executive Order 13211,
Actions Concerning Regulations That Significantly Affect Energy
Supply,
Distribution, or Use (66 FR 28355, May 22, 2001). This final rule
does
not contain any information collections subject to OMB approval
under
the Paperwork Reduction Act (PRA), 44 U.S.C. 3501 et seq., or impose
any enforceable duty or contain any unfunded mandate as described
under
Title II of the Unfunded Mandates Reform Act of 1995 (UMRA) (Public
Law
104-4). Nor does it require any special considerations under Executive
Order 12898, entitled Federal Actions to Address Environmental Justice
in Minority Populations and Low-Income Populations (59 FR 7629,
February 16, 1994); or OMB review or any Agency action under Executive
Order 13045, entitled Protection of Children from Environmental
Health
Risks and Safety Risks (62 FR 19885, April 23, 1997). This action
does
not involve any technical standards that would require Agency
consideration of voluntary consensus standards pursuant to section
12(d) of the National Technology Transfer and Advancement Act of
1995
(NTTAA), Public Law 104-113, section 12(d) (15 U.S.C. 272 note).
Since
tolerances and exemptions that are established on the basis of a
petition under section 408(d) of FFDCA, such as the tolerance in
this
final rule, do not require the issuance of a proposed rule, the
requirements of the Regulatory Flexibility Act (RFA) (5 U.S.C. 601
et
seq.) do not apply. In addition, the Agency has determined that
this
action will not have a substantial direct effect on States, on the
relationship between the national government and the States, or
on the
distribution of power and responsibilities among the various levels
of
government, as specified in Executive Order 13132, entitled
Federalism(64 FR 43255, August 10, 1999). Executive Order 13132
requires EPA to develop an accountable process to ensure ``meaningful
and timely input by State and local officials in the development
of
regulatory policies that have federalism implications.'' ``Policies
that have federalism implications'' is defined in the Executive
order
to include regulations that have ``substantial direct effects on
the
States, on the relationship between the national government and
the
States, or on the distribution of power and responsibilities among
the
various levels of government.'' This final rule directly regulates
growers, food processors, food handlers and food retailers, not
States.
This action does not alter the relationships or distribution of
power
and responsibilities established by Congress in the preemption
provisions of section 408(n)(4) of FFDCA. For these same reasons,
the
Agency has determined that this rule does not have any ``tribal
implications'' as described in Executive Order 13175, entitled
Consultation and Coordination with Indian Tribal Governments (65
FR
67249, November 6, 2000). Executive Order 13175, requires EPA to
develop an accountable process to ensure ``meaningful and timely
input
by tribal officials in the development of regulatory policies that
have
tribal implications.'' ``Policies that have tribal implications''
is
defined in the Executive order to include regulations that have
``substantial direct effects on one or more Indian tribes, on the
relationship between the Federal Government and the Indian tribes,
or
on the distribution of power and responsibilities between the Federal
Government and Indian tribes.'' This rule will not have substantial
direct effects on tribal governments, on the relationship between
the
Federal Government and Indian tribes, or on the distribution of
power
and responsibilities between the Federal Government and Indian tribes,
as specified in Executive Order 13175. Thus, Executive Order 13175
does
not apply to this rule.
VIII. Congressional Review Act
The Congressional Review Act, 5 U.S.C. 801 et seq.,
as added by the
Small Business Regulatory Enforcement Fairness Act of 1996, generally
provides that before a rule may take effect, the agency promulgating
the rule must submit a rule report, which includes a copy of the
rule,
to each House of the Congress and to the Comptroller General of
the
United States. EPA will submit a report containing this rule and
other
required information to the U.S. Senate, the U.S. House of
Representatives, and the Comptroller General of the United States
prior
to publication of this final rule in the Federal Register. This
final
rule is not a ``major rule'' as defined by 5 U.S.C. 804(2).
List of Subjects in 40 CFR Part 180
Environmental protection, Administrative practice and procedure,
Agricultural commodities, Pesticides and pests, Reporting and
recordkeeping requirements.
Dated: May 20, 2004.
James Jones,
Director, Office of Pesticide Programs.
• Therefore, 40 CFR chapter I is amended as follows:
PART 180--[AMENDED]
• 1. The authority citation for part 180 continues to read
as follows:
Authority: 21 U.S.C. 321(q), 346a and 371.
• 2. Section 180.598 is added to read as follows:
Sec. 180.598 Novaluron; tolerances for residues.
(a) General. Tolerances are established for
residues of the
insecticide novaluron, 1-[3-chloro-4-(1,1,2-trifluoro-2-trifluoro-
methoxyethoxy)phenyl]-3-(2,6-difluorobenzoyl)urea, in or on the
following raw agricultural commodities:
------------------------------------------------------------------------
Commodity Parts per million
------------------------------------------------------------------------
Apple, wet pomace.................................... 8.0
Cattle, fat.......................................... 11
Cattle, kidney....................................... 1.0
Cattle, liver........................................ 1.0
Cattle, meat......................................... 0.60
Cattle, meat byproducts, except liver and kidney..... 0.60
Cotton, gin byproducts............................... 30
Cotton, undelinted seed.............................. 0.60
Eggs................................................. 0.05
Fruit, pome, group 11................................ 2.0
Goat, fat............................................ 11
Goat, kidney......................................... 1.0
Goat, liver.......................................... 1.0
Goat, meat........................................... 0.60
Goat, meat byproducts except liver and kidney........ 0.60
Hog, fat............................................. 0.05
Hog, meat............................................ 0.01
Hog, meat byproducts................................. 0.01
Horse, fat........................................... 11
Horse, kidney........................................ 1.0
Horse, liver......................................... 1.0
Horse, meat.......................................... 0.60
Horse, meat byproducts, except liver and kidney...... 0.60
Milk................................................. 1.0
Milk, fat............................................ 20
Poultry, fat......................................... 0.40
Poultry, meat........................................ 0.03
Poultry, meat byproducts............................. 0.04
Sheep, fat........................................... 11
Sheep, kidney........................................ 1.0
Sheep, liver......................................... 1.0
Sheep, meat.......................................... 0.60
Sheep, meat byproducts, except liver and kidney...... 0.60
Vegetables, tuberous and corn, subgroup 1C........... 0.05
------------------------------------------------------------------------
[[Page 31022]]
(b) Section 18 emergency exemptions. [Reserved]
(c) Tolerances with regional registrations. [Reserved]
(d) Indirect or inadvertant residues. [Reserved]
[FR Doc. 04-12316 Filed 6-1-04; 8:45 am]
BILLING CODE 6560-50-S
|

